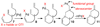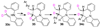Synthesis of polycyclic nitrogen heterocycles via alkene aminopalladation/carbopalladation cascade reactions
- PMID: 20141119
- PMCID: PMC2830278
- DOI: 10.1021/ol100033s
Synthesis of polycyclic nitrogen heterocycles via alkene aminopalladation/carbopalladation cascade reactions
Abstract
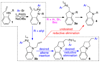
A new method for the synthesis of tricyclic nitrogen heterocycles from N,2-diallylaniline derivatives is described. These transformations proceed via sequential alkene aminopalladation of an intermediate L(n)Pd(Ar)(NRR') species followed by alkene carbopalladation of the resulting L(n)Pd(Ar)(R) complex. Both alkene insertion steps occur in preference to C-N or C-C bond-forming reductive elimination. An unusual 1,3-palladium shift occurs when 2-Allyl-N-(2-vinylphenyl)aniline is employed as substrate, which yields a tetracyclic molecule with three contiguous stereocenters.
Figures
References
-
-
Reviews: Wolfe JP. Eur. J. Org. Chem. 2007:571. Wolfe JP. Synlett. 2008:2913.
-
-
- Lira R, Wolfe JP. J. Am. Chem. Soc. 2004;126:13906. - PubMed
- Bertrand MB, Wolfe JP. Tetrahedron. 2005;61:6447.
-
- Ito Y, Nakajo E, Natatsuka M, Saegusa T. Tetrahedron Lett. 1983;24:2881.
- Pearson WH, Fang W-K. J. Org. Chem. 2000;65:7158. - PubMed
-
- Negishi E-i, Wang G, Zhu G. Top. Organomet. Chem. 2006;19:1.
- Maddaford SP, Andersen NG, Cristofoli WA, Keay BA. J. Am. Chem. Soc. 1996;118:10766.
- de Meijere A, Meyer FE. Angew. Chem. Int. Ed. Engl. 1994;33:2379.
- Zeni G, Larock RC. Chem. Rev. 2006;106:4644. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials