Novel targets for the treatment of autosomal dominant polycystic kidney disease
- PMID: 20141351
- PMCID: PMC2861144
- DOI: 10.1517/13543781003588491
Novel targets for the treatment of autosomal dominant polycystic kidney disease
Abstract
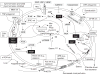
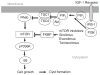
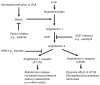
Importance of the field: Autosomal dominant (AD) polycystic kidney disease (PKD) is the most common life-threatening hereditary disorder. There is currently no therapy that slows or prevents cyst formation and kidney enlargement in humans. An increasing number of animal studies have advanced our understanding of molecular and cellular targets of PKD.
Areas covered in the review: The purpose of this review is to summarize the molecular and cellular targets involved in cystogenesis and to update on the promising therapies that are being developed and tested based on knowledge of these molecular and cellular targets.
What the reader will gain: Insight into the pathogenesis of PKD and how a better understanding of the pathogenesis of PKD has led to the development of potential therapies to inhibit cyst formation and/or growth and improve kidney function.
Take home message: The results of animal studies in PKD have led to the development of clinical trials testing potential new therapies to reduce cyst formation and/or growth. A vasopressin V2 receptor antagonist, mTOR inhibitors, blockade of the renin-angiotensin system and statins that reduce cyst formation and improve renal function in animal models of PKD are being tested in interventional studies in humans.
Figures
References
-
- Chapman AB, Guay-Woodford LM, Grantham JJ, et al. Renal structure in early autosomal-dominant polycystic kidney disease (ADPKD): The Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP) cohort. Kidney Int. 2003;64:1035–45. - PubMed
-
- Grantham JJ, Chapman AB, Torres VE. Volume progression in autosomal dominant polycystic kidney disease: the major factor determining clinical outcomes. Clin J Am Soc Nephrol. 2006;1:148–57. - PubMed
-
- Rossetti S, Harris PC. Genotype-phenotype correlations in autosomal dominant and autosomal recessive polycystic kidney disease. J Am Soc Nephrol. 2007;18(5):1374–80. - PubMed
-
- Harris PC. Autosomal dominant polycystic kidney disease: clues to pathogenesis. Hum Mol Genet. 1999;8:1861–186. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
