Facts, fancies and follies of drug-induced QT/QTc interval shortening
- PMID: 20141519
- PMCID: PMC2823353
- DOI: 10.1111/j.1476-5381.2009.00554.x
Facts, fancies and follies of drug-induced QT/QTc interval shortening
Abstract
Parallels exist between drug-induced QT/QTc prolongation and shortening. However, these parallels are largely superficial and the experience with drug-induced QTc prolongation and its potential proarrhythmic link cannot be directly applied to drug-related QTc shortening. The congenital short QT syndrome (SQTS) is clearly much less prevalent than congenital, long QT syndrome, possibly some 1000 times. If the same discrepancy exists between arrhythmic susceptibility to drug-induced QTc prolongation and shortening, it is questionable whether regulatory burden should be imposed on drugs that might cause serious arrhythmia, once in many millions of exposures. Further, majority of torsadegenic drugs block the IKr current which is susceptible to the drug blockade because of the corresponding channel geometry. There is no parallel known for drug-induced QTc shortening. Also, all drugs that prolong QTc interval massively cause torsade de pointes tachycardia in more than exceptional isolated instances. On the contrary, digitalis that causes substantial QTc shortening is not known to trigger frequently ventricular arrhythmias. Moreover, most available population QTc data were obtained with Bazett's correction which produces erroneous QTc shortening at slow heart rates. Safety limits derived from such data are inappropriate. Because practically all new drugs undergo the so-called thorough QT study, drug-induced QTc shortening will not go unnoticed for any new pharmaceutical. Describing drug-related QTc shortening in the label seems sufficient to avoid treatment of the rare SQTS subjects. Intensive investigations of QTc-shortening drugs (similar to those of drugs with positive thorough QT studies) do not seem to be warranted.
This article is a commentary on Shah, pp. 58–69 of this issue and is part of a themed section on QT safety. To view this issue visit
Figures
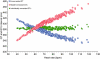
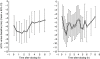
Comment on
-
Drug-induced QT interval shortening: potential harbinger of proarrhythmia and regulatory perspectives.Br J Pharmacol. 2010 Jan;159(1):58-69. doi: 10.1111/j.1476-5381.2009.00191.x. Epub 2009 Jun 25. Br J Pharmacol. 2010. PMID: 19563537 Free PMC article. Review.
Similar articles
-
Drug-induced at interval prolongation: clinical, safety and regulatory updaes.Curr Drug Saf. 2010 Jan;5(1):41-3. doi: 10.2174/157488610789869120. Curr Drug Saf. 2010. PMID: 20218963 No abstract available.
-
QT interval and drug therapy.Drug Ther Bull. 2016 Mar;54(3):33-6. doi: 10.1136/dtb.2016.3.0390. Drug Ther Bull. 2016. PMID: 26966121 Review.
-
[Drug induced QT prolongation].Wien Klin Wochenschr. 2008;120(5-6):128-35. doi: 10.1007/s00508-008-0940-6. Wien Klin Wochenschr. 2008. PMID: 18365152 Review. German.
-
QT interval prolongation: preclinical and clinical testing arrhythmogenesis in drugs and regulatory implications.Curr Drug Saf. 2010 Jan;5(1):54-7. doi: 10.2174/157488610789869148. Curr Drug Saf. 2010. PMID: 20210719
-
Predicting the Unpredictable: Drug-Induced QT Prolongation and Torsades de Pointes.J Am Coll Cardiol. 2016 Apr 5;67(13):1639-1650. doi: 10.1016/j.jacc.2015.12.063. J Am Coll Cardiol. 2016. PMID: 27150690 Review.
Cited by
-
Novel targets and regulatory ordeal by QT interval.Br J Clin Pharmacol. 2010 Apr;69(4):325-8. doi: 10.1111/j.1365-2125.2010.03657.x. Br J Clin Pharmacol. 2010. PMID: 20406216 Free PMC article. No abstract available.
-
Pharmacological and electrophysiological characterization of AZSMO-23, an activator of the hERG K(+) channel.Br J Pharmacol. 2015 Jun;172(12):3112-25. doi: 10.1111/bph.13115. Epub 2015 Apr 10. Br J Pharmacol. 2015. PMID: 25684549 Free PMC article.
-
Thorough QT Studies: Questions and Quandaries.Drug Saf. 2010 Jan 1;33(1):1-14. doi: 10.2165/11319160-000000000-00000. Drug Saf. 2010. PMID: 20000862 Review.
-
Drug-Induced QT/QTc Interval Shortening: Lessons from Drug-Induced QT/QTc Prolongation.Drug Saf. 2016 Jul;39(7):647-59. doi: 10.1007/s40264-016-0411-3. Drug Saf. 2016. PMID: 26968542 Review.
-
A thorough QTc study to evaluate the effects of oral rilzabrutinib administered alone and with ritonavir in healthy subjects.Clin Transl Sci. 2022 Jun;15(6):1507-1518. doi: 10.1111/cts.13271. Epub 2022 Apr 3. Clin Transl Sci. 2022. PMID: 35301810 Free PMC article. Clinical Trial.
References
-
- Ahnve S. QT interval prolongation in acute myocardial infarction. Eur Heart J. 1985;6:85–95. - PubMed
-
- Asami T, Suzuki H, Yazaki S, Sato S, Uchiyama M. Effects of thyroid hormone deficiency on electrocardiogram findings of congenitally hypothyroid neonates. Thyroid. 2001;11:765–768. - PubMed
-
- Baig MK, Goldman JH, Caforio AL, Coonar AS, Keeling PJ, McKenna WJ. Familial dilated cardiomyopathy: cardiac abnormalities are common in asymptomatic relatives and may represent early disease. J Am Coll Cardiol. 1998;31:195–201. - PubMed
-
- Codd MB, Sugrue DD, Gersh BJ, Melton LJ. Epidemiology of idiopathic dilated and hypertrophic cardiomyopathy. Circulation. 1989;80:564–572. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

