Differential impact of familial hypercholesterolemia and combined hyperlipidemia on vascular wall and network remodeling in mice
- PMID: 20141600
- PMCID: PMC2952832
- DOI: 10.1111/j.1549-8719.2009.00003.x
Differential impact of familial hypercholesterolemia and combined hyperlipidemia on vascular wall and network remodeling in mice
Abstract
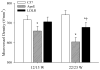
Genetic familial hypercholesterolemia (FH) and combined hyperlipidemia (FCH) are characterized by elevated plasma low-density lipoprotein (LDL) (FH) and LDL/triglycerides (FCH), with mouse models represented by LDL receptor (LDLR) and apolipoprotein E (ApoE) gene deletion mice, respectively. Given the impact of FH and FCH on health outcomes, we determined the impact of FH/FCH on vascular structure in LDLR and ApoE mice. LDLR, ApoE and control mice were utilized at 12-13 and 22-23 weeks when gracilis arteries were studied for wall mechanics and gastrocnemius muscles were harvested for microvessel density measurements. Conduit arteries and plasma samples were harvested for biochemical analyses. Arteries from ApoE and LDLR exhibited blunted expansion versus control, reduced distensibility and left-shifted stress versus strain relation (LDLR > ApoE). Microvessel density was reduced in ApoE and LDLR (ApoE > LDLR). Secondary analyses suggested that wall remodeling in LDLR was associated with cholesterol and MCP-1, while rarefaction in ApoE was associated with tumor necrosis factors-alpha, triglycerides and vascular production of TxA(2). Remodeling in ApoE and LDLR appears distinct; as that in LDLR is preferential for vascular walls, while that for ApoE is stronger for rarefaction. Remodeling in LDLR may be associated with cellular adhesion, while that in ApoE may be associated with pro-apoptotsis and constrictor prostanoid generation.
Figures
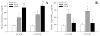




Similar articles
-
Increased arachidonic acid-induced thromboxane generation impairs skeletal muscle arteriolar dilation with genetic dyslipidemia.Microcirculation. 2008 Oct;15(7):621-31. doi: 10.1080/10739680802308334. Microcirculation. 2008. PMID: 18720229 Free PMC article.
-
Frequency of low-density lipoprotein receptor gene mutations in patients with a clinical diagnosis of familial combined hyperlipidemia in a clinical setting.J Am Coll Cardiol. 2008 Nov 4;52(19):1546-53. doi: 10.1016/j.jacc.2008.06.050. J Am Coll Cardiol. 2008. PMID: 19007590
-
Altered mechanisms of endothelium-dependent dilation in skeletal muscle arterioles with genetic hypercholesterolemia.Am J Physiol Regul Integr Comp Physiol. 2007 Sep;293(3):R1110-9. doi: 10.1152/ajpregu.00410.2007. Epub 2007 Jul 11. Am J Physiol Regul Integr Comp Physiol. 2007. PMID: 17626122
-
Understanding hyperlipidemia and atherosclerosis: lessons from genetically modified apoe and ldlr mice.Clin Chem Lab Med. 2005;43(5):470-9. doi: 10.1515/CCLM.2005.085. Clin Chem Lab Med. 2005. PMID: 15899668 Review.
-
Gene therapy for familial hypercholesterolemia.Curr Pharm Des. 2011;17(24):2575-91. doi: 10.2174/138161211797247550. Curr Pharm Des. 2011. PMID: 21774774 Review.
Cited by
-
Severe familial hypercholesterolemia impairs the regulation of coronary blood flow and oxygen supply during exercise.Basic Res Cardiol. 2016 Nov;111(6):61. doi: 10.1007/s00395-016-0579-9. Epub 2016 Sep 13. Basic Res Cardiol. 2016. PMID: 27624732 Free PMC article.
-
Role of adropin in arterial stiffening associated with obesity and type 2 diabetes.Am J Physiol Heart Circ Physiol. 2022 Nov 1;323(5):H879-H891. doi: 10.1152/ajpheart.00385.2022. Epub 2022 Sep 9. Am J Physiol Heart Circ Physiol. 2022. PMID: 36083795 Free PMC article.
-
Crossroads between peripheral atherosclerosis, western-type diet and skeletal muscle pathophysiology: emphasis on apolipoprotein E deficiency and peripheral arterial disease.J Biomed Sci. 2017 Jul 8;24(1):42. doi: 10.1186/s12929-017-0346-8. J Biomed Sci. 2017. PMID: 28688452 Free PMC article. Review.
-
Coronary microvascular obstruction and dysfunction in patients with acute myocardial infarction.Nat Rev Cardiol. 2024 May;21(5):283-298. doi: 10.1038/s41569-023-00953-4. Epub 2023 Nov 24. Nat Rev Cardiol. 2024. PMID: 38001231 Review.
-
Heart of the matter: coronary dysfunction in metabolic syndrome.J Mol Cell Cardiol. 2012 Apr;52(4):848-56. doi: 10.1016/j.yjmcc.2011.06.025. Epub 2011 Jul 13. J Mol Cell Cardiol. 2012. PMID: 21767548 Free PMC article. Review.
References
-
- Aggarwal NT, Pfister SL, Campbell WB. Hypercholesterolemia enhances 15-lipoxygenase-mediated vasorelaxation and acetylcholine-induced hypotension. Arterioscler Thromb Vasc Biol. 2008;28(12):2209–15. - PubMed
-
- Aggoun Y, Bonnet D, Sidi D, Girardet JP, Brucker E, Polak M, Safar ME, Levy BI. Arterial mechanical changes in children with familial hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2000;20(9):2070–5. - PubMed
-
- Austin MA, Hutter CM, Zimmern RL, Humphries SE. Genetic causes of monogenic heterozygous familial hypercholesterolemia: a HuGE prevalence review. Am J Epidemiol. 2004;160(5):407–20. - PubMed
-
- Benndorf RA, Schwedhelm E, Gnann A, Taheri R, Kom G, Didié M, Steenpass A, Ergün S, Böger RH. Isoprostanes inhibit vascular endothelial growth factor-induced endothelial cell migration, tube formation, and cardiac vessel sprouting in vitro, as well as angiogenesis in vivo via activation of the thromboxane A(2) receptor: a potential link between oxidative stress and impaired angiogenesis. Circ Res. 2008;103(9):1037–46. - PubMed
-
- Blanco-Colio LM, Martín-Ventura JL, de Teresa E, Farsang C, Gaw A, Gensini G, Leiter LA, Langer A, Martineau P, Egido J ACTFAST investigators. Elevated ICAM-1 and MCP-1 plasma levels in subjects at high cardiovascular risk are diminished by atorvastatin treatment. Atorvastatin on Inflammatory Markers study: a substudy of Achieve Cholesterol Targets Fast with Atorvastatin Stratified Titration. Am Heart J. 2007;153(5):881–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

