Endogenous n-3 fatty acids protect ovariectomy induced bone loss by attenuating osteoclastogenesis
- PMID: 20141608
- PMCID: PMC2855756
- DOI: 10.1111/j.1582-4934.2009.00649.x
Endogenous n-3 fatty acids protect ovariectomy induced bone loss by attenuating osteoclastogenesis
Abstract
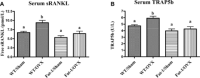
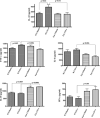
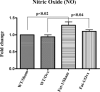
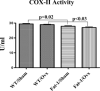
Beneficial effects of n-3 fatty acids (FA) on bone mineral density (BMD) have been reported in mice, rats and human beings, but the precise mechanisms involved have not been described. This study used the Fat-1 mouse, a transgenic model that synthesizes n-3 FA from n-6 FA to directly determine if outcome of bone health were correlated with n-3 FA. Ovariectomized (Ovx) and sham operated wild-type (WT) and Fat-1 mice were fed an AIN-93M diet containing 10% corn oil for 24 weeks. BMD was analysed by dual energy x-ray absorptiometry. Fat-1 Ovx mice exhibited significantly lower level of osteotropic factors like receptor activator of NF-kappaB ligand and tartrate-resistant acid phosphatase (TRAP)5b in serum and higher BMD in distal femoral metaphysis, proximal tibial metaphysis, femoral diaphysis and lumbar vertebra as compared to WT Ovx mice. LPS-stimulated bone marrow (BM) cells from Fat-1 Ovx mice produced significantly lower level of pro-inflammatory cytokines like tumour necrosis factor-alpha, interleukin (IL)-1-beta, IL-6 and higher level of anti-inflammatory cytokines like IL-10, IFN-gamma and higher level of nitric oxide as compared to BM cells from WT Ovx mice. LPS-stimulated COX-II activity as well as NF-kappaB activation in BM cells from Fat-1 Ovx mice was significantly less as compared to BM cells from WT Ovx mice. Furthermore, Fat-1 BM cells generated significantly less number of TRAP osteoclast-like cells as compared to WT BM cells. In conclusion, we offer further insight into the mechanisms involved in preventing the BMD loss in Ovx mice by n-3 FA using a Fat-1 transgenic mouse model.
Keywords: bone mineral density; inflammation; n-3 fatty acids; osteoclasts; osteoporosis.
Figures

Similar articles
-
Dietary n-3 fatty acids decrease osteoclastogenesis and loss of bone mass in ovariectomized mice.J Bone Miner Res. 2003 Jul;18(7):1206-16. doi: 10.1359/jbmr.2003.18.7.1206. J Bone Miner Res. 2003. PMID: 12854830
-
Conjugated linoleic Acid prevents ovariectomy-induced bone loss in mice by modulating both osteoclastogenesis and osteoblastogenesis.Lipids. 2014 Mar;49(3):211-24. doi: 10.1007/s11745-013-3872-5. Epub 2013 Dec 13. Lipids. 2014. PMID: 24338525 Free PMC article.
-
Short- and long-term effects of calcium and exercise on bone mineral density in ovariectomized rats.Br J Nutr. 2001 Oct;86(4):521-7. doi: 10.1079/bjn2001428. Br J Nutr. 2001. PMID: 11591240
-
Hydrogen gas protects against ovariectomy-induced osteoporosis by inhibiting NF-κB activation.Menopause. 2019 Jul;26(7):785-792. doi: 10.1097/GME.0000000000001310. Menopause. 2019. PMID: 31083022
-
Interleukin-7 influences osteoclast function in vivo but is not a critical factor in ovariectomy-induced bone loss.J Bone Miner Res. 2006 May;21(5):695-702. doi: 10.1359/jbmr.060117. J Bone Miner Res. 2006. PMID: 16734384
Cited by
-
Unraveling the Omega-3 Puzzle: Navigating Challenges and Innovations for Bone Health and Healthy Aging.Mar Drugs. 2024 Sep 28;22(10):446. doi: 10.3390/md22100446. Mar Drugs. 2024. PMID: 39452854 Free PMC article. Review.
-
Regulation of Cholesterol Metabolism in Liver: Link to NAFLD and Impact of n-3 PUFAs.J Lifestyle Med. 2013 Mar;3(1):19-25. Epub 2013 Mar 31. J Lifestyle Med. 2013. PMID: 26064833 Free PMC article. Review.
-
Skeletal effects of the saturated 3-thia Fatty Acid tetradecylthioacetic Acid in rats.PPAR Res. 2011;2011:436358. doi: 10.1155/2011/436358. Epub 2011 Dec 5. PPAR Res. 2011. PMID: 22190907 Free PMC article.
-
The fat-1 transgene in mice increases antioxidant potential, reduces pro-inflammatory cytokine levels, and enhances PPAR-gamma and SIRT-1 expression on a calorie restricted diet.Oxid Med Cell Longev. 2009 Nov-Dec;2(5):307-16. doi: 10.4161/oxim.2.5.9579. Oxid Med Cell Longev. 2009. PMID: 20716918 Free PMC article.
-
Endogenous ω-3 polyunsaturated fatty acid production confers resistance to obesity, dyslipidemia, and diabetes in mice.Mol Endocrinol. 2014 Aug;28(8):1316-28. doi: 10.1210/me.2014-1011. Epub 2014 Jun 30. Mol Endocrinol. 2014. PMID: 24978197 Free PMC article.
References
-
- Rosen CJ. Clinical practice. Postmenopausal osteoporosis. N Engl J Med . 2005; 353: 595–603. - PubMed
-
- Lacey JV Jr, Mink PJ, Lubin JH, et al . Menopausal hormone replacement therapy and risk of ovarian cancer. Jama . 2002; 288: 334–41. - PubMed
-
- Das UN. Essential fatty acids and osteoporosis. Nutrition . 2000; 16: 386–90. - PubMed
-
- Kruger MC, Coetzer H, de Winter R, et al . Calcium, gamma‐linolenic acid and eicosapentaenoic acid supplementation in senile osteoporosis. Aging . 1998; 10: 385–94. - PubMed
-
- Watkins BA, Lippman HE, Le Bouteiller L, et al . Bioactive fatty acids: role in bone biology and bone cell function. Prog Lipid Res . 2001; 40: 125–48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

