Calcium and ER stress mediate hepatic apoptosis after burn injury
- PMID: 20141609
- PMCID: PMC2855735
- DOI: 10.1111/j.1582-4934.2009.00644.x
Calcium and ER stress mediate hepatic apoptosis after burn injury
Abstract
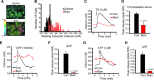
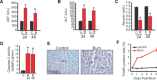
A hallmark of the disease state following severe burn injury is decreased liver function, which results in gross metabolic derangements that compromise patient survival. The underlying mechanisms leading to hepatocyte dysfunction after burn are essentially unknown. The aim of the present study was to determine the underlying mechanisms leading to hepatocyte dysfunction and apoptosis after burn. Rats were randomized to either control (no burn) or burn (60% total body surface area burn) and sacrificed at various time-points. Liver was either perfused to isolate primary rat hepatocytes, which were used for in vitro calcium imaging, or liver was harvested and processed for immunohistology, transmission electron microscopy, mitochondrial isolation, mass spectroscopy or Western blotting to determine the hepatic response to burn injury in vivo. We found that thermal injury leads to severely depleted endoplasmic reticulum (ER) calcium stores and consequent elevated cytosolic calcium concentrations in primary hepatocytes in vitro. Burn-induced ER calcium depletion caused depressed hepatocyte responsiveness to signalling molecules that regulate hepatic homeostasis, such as vasopressin and the purinergic agonist ATP. In vivo, thermal injury resulted in activation of the ER stress response and major alterations in mitochondrial structure and function - effects which may be mediated by increased calcium release by inositol 1,4,5-trisphosphate receptors. Our results reveal that thermal injury leads to dramatic hepatic disturbances in calcium homeostasis and resultant ER stress leading to mitochondrial abnormalities contributing to hepatic dysfunction and apoptosis after burn injury.
Keywords: ER stress; apoptosis; calcium; liver; thermal injury; unfolded protein response.
Figures


References
-
- Brigham PA, McLoughlin E. Burn incidence and medical care use in the United States: estimates, trends and data sources. J Burn Care Rehabil. 1996; 17: 95–107. - PubMed
-
- World Health Organization (WHO) . A graphical overview of the global burden of injuries. The injury chart book. Volume 29. Geneva : WHO ; 2002.
-
- Herndon DN, Tompkins RG. Support of the metabolic response to burn injury. Lancet. 2004; 363: 1895–902. - PubMed
-
- Herndon DN, Hart DW, Wolf SE, et al . Reversal of catabolism by beta‐blockade after severe burns. N Engl J Med. 2001; 345: 1223–9. - PubMed
-
- van den Berge G, Wouters P, Weekers F, et al . Intensive insulin therapy in critically ill patients. New Engl J Med. 2001; 345: 1359–67. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

