Calcium sensing receptor-dependent and receptor-independent activation of osteoblast replication and survival by strontium ranelate
- PMID: 20141614
- PMCID: PMC9181364
- DOI: 10.1111/j.1582-4934.2009.00673.x
Calcium sensing receptor-dependent and receptor-independent activation of osteoblast replication and survival by strontium ranelate
Abstract
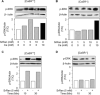
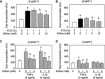
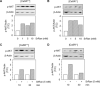
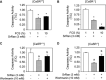
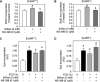
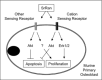
Age-related osteopenia is characterized by a negative balance between bone resorption and formation. The anti-osteoporotic drug strontium ranelate was found to reduce bone resorption and to promote bone formation. Here, we investigated the implication of the calcium-sensing receptor (CaSR) in the response to strontium ranelate using osteoblasts from CaSR knockout [CaSR(-/-)] and wild-type [CaSR(+/+)] mice. We showed that calcium and strontium ranelates increased cell replication in [CaSR(-/-)] and [CaSR(+/+)] osteoblasts. Strontium ranelate rapidly increased ERK1/2 phosphorylation in [CaSR(+/+)] but not in [CaSR(-/-)] osteoblasts, indicating that strontium ranelate can act independent of the CaSR/ERK1/2 cascade to promote osteoblast replication. We also showed that strontium ranelate prevented cell apoptosis induced by serum deprivation or the pro-inflammatory cytokines IL-1beta and TNF-alpha in [CaSR(-/-)] and [CaSR(+/+)] osteoblasts, indicating that CaSR is not the only receptor involved in the protective effect of strontium ranelate on osteoblast apoptosis. Strontium ranelate activated the Akt pro-survival pathway in [CaSR(-/-)] and [CaSR(+/+)] osteoblasts, and pharmacological inhibition of Akt abrogated the anti-apoptotic effect of strontium ranelate. Furthermore, both the proliferative and anti-apoptotic effects of strontium ranelate in [CaSR(-/-)] and [CaSR(+/+)] osteoblasts were abrogated by selective inhibition of COX-2. The results provide genetic and biochemical evidence that the effects of strontium ranelate on osteoblast replication and survival involve ERK1/2 and Akt signalling and PGE2 production, independent of CaSR expression. The finding that CaSR-dependent and CaSR-independent pathways mediate the beneficial effects of strontium ranelate on osteoblasts, provides novel insight into the mechanism of action of this anti-osteoporotic agent on osteoblastogenesis.
Figures

Similar articles
-
Osteoblasts play key roles in the mechanisms of action of strontium ranelate.Br J Pharmacol. 2009 Aug;157(7):1291-300. doi: 10.1111/j.1476-5381.2009.00305.x. Epub 2009 Jun 25. Br J Pharmacol. 2009. PMID: 19563530 Free PMC article.
-
Activation of FGF receptors is a new mechanism by which strontium ranelate induces osteoblastic cell growth.Cell Physiol Biochem. 2011;27(3-4):243-50. doi: 10.1159/000327950. Epub 2011 Apr 1. Cell Physiol Biochem. 2011. PMID: 21471713
-
The Calcium-sensing Receptor (CaR) is involved in strontium ranelate-induced osteoblast differentiation and mineralization.Horm Metab Res. 2010 Aug;42(9):627-31. doi: 10.1055/s-0030-1255091. Epub 2010 Jun 17. Horm Metab Res. 2010. PMID: 20560105
-
Strontium ranelate in osteoporosis.Curr Pharm Des. 2002;8(21):1907-16. doi: 10.2174/1381612023393639. Curr Pharm Des. 2002. PMID: 12171530 Review.
-
The calcium-sensing receptor in bone metabolism: from bench to bedside and back.Osteoporos Int. 2015 Aug;26(8):2055-71. doi: 10.1007/s00198-015-3203-1. Epub 2015 Jun 23. Osteoporos Int. 2015. PMID: 26100412 Review.
Cited by
-
Effects of strontium ions with potential antibacterial activity on in vivo bone regeneration.Sci Rep. 2021 Apr 22;11(1):8745. doi: 10.1038/s41598-021-88058-1. Sci Rep. 2021. PMID: 33888790 Free PMC article.
-
Facilitated and Controlled Strontium Ranelate Delivery Using GCS-HA Nanocarriers Embedded into PEGDA Coupled with Decortication Driven Spinal Regeneration.Int J Nanomedicine. 2021 Jun 22;16:4209-4224. doi: 10.2147/IJN.S274461. eCollection 2021. Int J Nanomedicine. 2021. PMID: 34188470 Free PMC article.
-
Strontium ranelate: a review of its use in the treatment of postmenopausal osteoporosis.Drugs. 2010 Apr 16;70(6):733-59. doi: 10.2165/10481900-000000000-00000. Drugs. 2010. PMID: 20394457 Review.
-
Effects of 0.01 mM strontium on human periodontal ligament stem cell osteogenic differentiation via the Wnt/β-catenin signaling pathway.J Int Med Res. 2025 Feb;53(2):3000605251315024. doi: 10.1177/03000605251315024. J Int Med Res. 2025. PMID: 39932304 Free PMC article.
-
Signaling pathways affecting skeletal health.Curr Osteoporos Rep. 2012 Sep;10(3):190-8. doi: 10.1007/s11914-012-0109-0. Curr Osteoporos Rep. 2012. PMID: 22711369 Review.
References
-
- Riggs BL, Parfitt AM. Drugs used to treat osteoporosis: the critical need for a uniform nomenclature based on their action on bone remodeling. J Bone Miner Res . 2005; 20: 177–84. - PubMed
-
- Meunier PJ, Roux C, Seeman E, et al . The effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis. N Engl J Med . 2004; 350: 459–68. - PubMed
-
- Reginster JY, Seeman E, De Vernejoul MC, et al . Strontium ranelate reduces the risk of nonvertebral fractures in postmenopausal women with osteoporosis: Treatment of Peripheral Osteoporosis (TROPOS) study. J Clin Endocrinol Metab . 2005; 90: 2816–22. - PubMed
-
- Marie PJ. Strontium as therapy for osteoporosis. Curr Opin Pharmaco . 2005; 5: 633–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

