Characterization of the S-denitrosylating activity of bilirubin
- PMID: 20141617
- PMCID: PMC9181359
- DOI: 10.1111/j.1582-4934.2009.00680.x
Characterization of the S-denitrosylating activity of bilirubin
Abstract
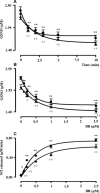
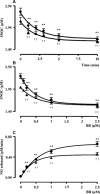
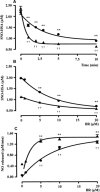
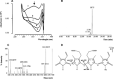
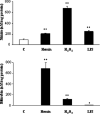
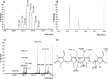
Bilirubin-IX-alpha (BR) is an endogenous molecule with a strong antioxidant feature due to its ability to scavenge free radicals. In this paper, we demonstrated that BR, at concentrations close to those found within the cell (0.1-2.5 microM), acted as a denitrosylating agent and increased the release of nitric oxide from S-nitrosoglutathione (GSNO) and S-nitrosocysteine (SNOC) (2.5 microM). The complexation of BR with saturating concentrations of human serum albumin (HSA, 2.5 microM) did not further increase nitric oxide release from GSNO and SNOC. At concentrations similar to those reached in plasma (5-20 microM), BR denitrosylated S-nitroso-HSA (2.5 microM), the main circulating S-nitrosothiol, and this effect was potentiated by the complexation of BR with saturating HSA (20 microM). Furthermore, the product(s) of the reaction between nitric oxide and BR were identified. Ultraviolet and mass spectrometry analysis revealed that nitric oxide binds to BR forming a N-nitroso derivative (BR-nitric oxide) with extinction coefficients of 1.393 mM(-1)cm(-1) and 2.254 mM(-1)cm(-1) in methanol and NaOH, respectively. The formation of BR-nitric oxide did not occur only in a reconstituted system, but was confirmed in rat fibroblasts exposed to pro-oxidant stimuli. These results provided novel insights on the antioxidant characteristic of BR through its interaction with nitric oxide, a gaseous neurotransmitter with a well-known dual effect, namely neuroprotective under physiological conditions or neurotoxic if produced in excess, and proposed BR-nitric oxide as a new biomarker of oxidative/nitrosative stress.
Figures
References
-
- Kutty RK, Maines MD. Purification and characterization of biliverdin reductase from rat liver. J Biol Chem . 1981; 256: 3956–62. - PubMed
-
- Maines MD. Heme oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J . 1988; 2: 2557–68. - PubMed
-
- Kapitulnik J. Bilirubin: an endogenous product of heme degradation with both cytotoxic and cytoprotective properties. Mol Pharmacol . 2004; 66: 773–9. - PubMed
-
- Ostrow JD, Pascolo L, Shapiro SM, et al . New concepts in bilirubin encephalopathy. Eur J Clin Invest . 2003; 33: 988–97. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

