Electrostatic tuning of cellular excitability
- PMID: 20141752
- PMCID: PMC2814211
- DOI: 10.1016/j.bpj.2009.10.026
Electrostatic tuning of cellular excitability
Abstract
Voltage-gated ion channels regulate the electric activity of excitable tissues, such as the heart and brain. Therefore, treatment for conditions of disturbed excitability is often based on drugs that target ion channels. In this study of a voltage-gated K channel, we propose what we believe to be a novel pharmacological mechanism for how to regulate channel activity. Charged lipophilic substances can tune channel opening, and consequently excitability, by an electrostatic interaction with the channel's voltage sensors. The direction of the effect depends on the charge of the substance. This was shown by three compounds sharing an arachidonyl backbone but bearing different charge: arachidonic acid, methyl arachidonate, and arachidonyl amine. Computer simulations of membrane excitability showed that small changes in the voltage dependence of Na and K channels have prominent impact on excitability and the tendency for repetitive firing. For instance, a shift in the voltage dependence of a K channel with -5 or +5 mV corresponds to a threefold increase or decrease in K channel density, respectively. We suggest that electrostatic tuning of ion channel activity constitutes a novel and powerful pharmacological approach with which to affect cellular excitability.
Copyright (c) 2010 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures


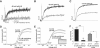

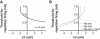
Similar articles
-
Lipoelectric modification of ion channel voltage gating by polyunsaturated fatty acids.Biophys J. 2008 Sep;95(5):2242-53. doi: 10.1529/biophysj.108.130757. Epub 2008 May 23. Biophys J. 2008. PMID: 18502799 Free PMC article.
-
More gating charges are needed to open a Shaker K+ channel than are needed to open an rBIIA Na+ channel.Biophys J. 2008 Aug;95(3):1165-75. doi: 10.1529/biophysj.108.130765. Epub 2008 Apr 4. Biophys J. 2008. PMID: 18390620 Free PMC article.
-
Atomic mutagenesis in ion channels with engineered stoichiometry.Elife. 2016 Oct 6;5:e18976. doi: 10.7554/eLife.18976. Elife. 2016. PMID: 27710770 Free PMC article.
-
Function and mechanism of axonal targeting of voltage-sensitive potassium channels.Prog Neurobiol. 2011 Jul;94(2):115-32. doi: 10.1016/j.pneurobio.2011.04.009. Epub 2011 Apr 22. Prog Neurobiol. 2011. PMID: 21530607 Free PMC article. Review.
-
Electrical excitability of cancer cells-CELEX model updated.Cancer Metastasis Rev. 2024 Dec;43(4):1579-1591. doi: 10.1007/s10555-024-10195-6. Epub 2024 Jul 8. Cancer Metastasis Rev. 2024. PMID: 38976181 Free PMC article. Review.
Cited by
-
Failure of delayed nonsynaptic neuronal plasticity underlies age-associated long-term associative memory impairment.BMC Neurosci. 2012 Aug 17;13:103. doi: 10.1186/1471-2202-13-103. BMC Neurosci. 2012. PMID: 22898271 Free PMC article.
-
Atom-by-atom tuning of the electrostatic potassium-channel modulator dehydroabietic acid.J Gen Physiol. 2018 May 7;150(5):731-750. doi: 10.1085/jgp.201711965. Epub 2018 Apr 6. J Gen Physiol. 2018. PMID: 29626041 Free PMC article.
-
Resin-acid derivatives as potent electrostatic openers of voltage-gated K channels and suppressors of neuronal excitability.Sci Rep. 2015 Aug 24;5:13278. doi: 10.1038/srep13278. Sci Rep. 2015. PMID: 26299574 Free PMC article.
-
Coupling stabilizers open KV1-type potassium channels.Proc Natl Acad Sci U S A. 2020 Oct 27;117(43):27016-27021. doi: 10.1073/pnas.2007965117. Epub 2020 Oct 13. Proc Natl Acad Sci U S A. 2020. PMID: 33051293 Free PMC article.
-
The conserved phenylalanine in the K+ channel voltage-sensor domain creates a barrier with unidirectional effects.Biophys J. 2013 Jan 8;104(1):75-84. doi: 10.1016/j.bpj.2012.11.3827. Epub 2013 Jan 8. Biophys J. 2013. PMID: 23332060 Free PMC article.
References
-
- Jentsch T.J. Neuronal KCNQ potassium channels: physiology and role in disease. Nat. Rev. Neurosci. 2000;1:21–30. - PubMed
-
- Jespersen T., Grunnet M., Olesen S.P. The KCNQ1 potassium channel: from gene to physiological function. Physiology (Bethesda) 2005;20:408–416. - PubMed
-
- Robbins J. KCNQ potassium channels: physiology, pathophysiology, and pharmacology. Pharmacol. Ther. 2001;90:1–19. - PubMed
-
- Börjesson S.I., Elinder F. Structure, function, and modification of the voltage sensor in voltage-gated ion channels. Cell Biochem. Biophys. 2008;52:149–174. - PubMed
-
- Long S.B., Campbell E.B., Mackinnon R. Voltage sensor of Kv1.2: structural basis of electromechanical coupling. Science. 2005;309:903–908. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

