Electrostatic solvation energy for two oppositely charged ions in a solvated protein system: salt bridges can stabilize proteins
- PMID: 20141761
- PMCID: PMC2814203
- DOI: 10.1016/j.bpj.2009.10.031
Electrostatic solvation energy for two oppositely charged ions in a solvated protein system: salt bridges can stabilize proteins
Abstract
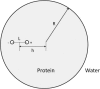
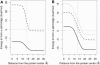
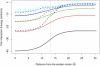
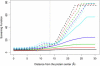
Born-type electrostatic continuum methods have been an indispensable ingredient in a variety of implicit-solvent methods that reduce computational effort by orders of magnitude compared to explicit-solvent MD simulations and thus enable treatment using larger systems and/or longer times. An analysis of the limitations and failures of the Born approaches serves as a guide for fundamental improvements without diminishing the importance of prior works. One of the major limitations of the Born theory is the lack of a liquidlike description of the response of solvent dipoles to the electrostatic field of the solute and the changes therein, a feature contained in the continuum Langevin-Debye (LD) model applied here to investigate how Coulombic interactions depend on the location of charges relative to the protein/water boundary. This physically more realistic LD model is applied to study the stability of salt bridges. When compared head to head using the same (independently measurable) physical parameters (radii, dielectric constants, etc.), the LD model is in good agreement with observations, whereas the Born model is grossly in error. Our calculations also suggest that a salt bridge on the protein's surface can be stabilizing when the charge separation is < or =4 A.
Copyright (c) 2010 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Interactions of macromolecules with salt ions: an electrostatic theory for the Hofmeister effect.Proteins. 2005 Oct 1;61(1):69-78. doi: 10.1002/prot.20500. Proteins. 2005. PMID: 16044460
-
Charged patchy particle models in explicit salt: Ion distributions, electrostatic potentials, and effective interactions.J Chem Phys. 2015 Aug 14;143(6):064904. doi: 10.1063/1.4928077. J Chem Phys. 2015. PMID: 26277163
-
Langevin-Debye model for nonlinear electrostatic screening of solvated ions.Phys Rev Lett. 2009 Feb 6;102(5):057603. doi: 10.1103/PhysRevLett.102.057603. Epub 2009 Feb 6. Phys Rev Lett. 2009. PMID: 19257555
-
Protein stabilization by salt bridges: concepts, experimental approaches and clarification of some misunderstandings.J Mol Recognit. 2004 Jan-Feb;17(1):1-16. doi: 10.1002/jmr.657. J Mol Recognit. 2004. PMID: 14872533 Review.
-
Significant role of electrostatic interactions for stabilization of protein assemblies.Adv Biophys. 1997;34:41-54. doi: 10.1016/s0065-227x(97)89630-x. Adv Biophys. 1997. PMID: 9204125 Review.
Cited by
-
Structural basis of p38α regulation by hematopoietic tyrosine phosphatase.Nat Chem Biol. 2011 Nov 6;7(12):916-24. doi: 10.1038/nchembio.707. Nat Chem Biol. 2011. PMID: 22057126 Free PMC article.
-
Resting and active states of the ERK2:HePTP complex.J Am Chem Soc. 2011 Nov 2;133(43):17138-41. doi: 10.1021/ja2075136. Epub 2011 Oct 10. J Am Chem Soc. 2011. PMID: 21985012 Free PMC article.
-
The energetic contribution of induced electrostatic asymmetry to DNA bending by a site-specific protein.J Mol Biol. 2011 Feb 18;406(2):285-312. doi: 10.1016/j.jmb.2010.12.012. Epub 2010 Dec 15. J Mol Biol. 2011. PMID: 21167173 Free PMC article.
References
-
- Cramer C.J., Truhlar D.G. Implicit solvation models: equilibria, structure, spectra, and dynamics. Chem. Rev. 1999;99:2161–2200. - PubMed
-
- Sandberg L., Edholm O. Nonlinear response effects in continuum models of the hydration of ions. J. Chem. Phys. 2002;116:2936–2944.
-
- Bucher M., Porter T.L. Analysis of the Born model for hydration of ions. J. Phys. Chem. 1986;90:3406–3411.
-
- Kakitani T., Mataga N. On the possibility of dielectric saturation in molecular systems. Chem. Phys. Lett. 1986;124:437–441.
-
- Sandberg L., Edholm O. A fast and simple method to calculate protonation states in proteins. Proteins. 1999;36:474–483. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

