In vivo long-term synaptic plasticity of glial cells
- PMID: 20142269
- PMCID: PMC2852994
- DOI: 10.1113/jphysiol.2009.178988
In vivo long-term synaptic plasticity of glial cells
Abstract
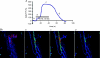
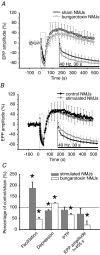
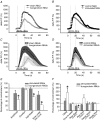
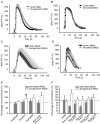
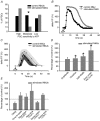
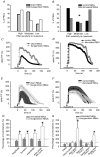
Evidence showing the ability of glial cells to detect, respond to and modulate synaptic transmission and plasticity has contributed to the notion of glial cells as active synaptic partners. However, synaptically induced plasticity of glia themselves remains ill defined. Here we used the amphibian neuromuscular junction (NMJ) to study plasticity of perisynaptic Schwann cells (PSCs), glial cells at this synapse, following long-term in vivo modifications of synaptic activity. We used two models that altered synaptic activity in different manners. First, chronic blockade of postsynaptic nicotinic receptors using alpha-bungarotoxin (alpha-BTx) decreased facilitation, increased synaptic depression and decreased post-tetanic potentiation (PTP). Second, chronic nerve stimulation increased facilitation and resistance to synaptic depression, while leaving PTP unaltered. Our results indicate that there is no direct relationship between transmitter release and PSC calcium responses. Indeed, despite changes in transmitter release and plasticity in stimulated NMJs, nerve-evoked PSC calcium responses were similar to control. Similarly, PSC calcium responses in alpha-BTx treated NMJs were delayed and smaller in amplitude, even though basal level of transmitter release was increased. Also, when isolating purinergic and muscarinic components of PSC calcium responses, we found an increased sensitivity to ATP and a decreased sensitivity to muscarine in chronically stimulated NMJs. Conversely, in alpha-BTx treated NMJs, PSC sensitivity remained unaffected, but ATP- and muscarine-induced calcium responses were prolonged. Thus, our results reveal complex modifications of PSC properties, with differential modulation of signalling pathways that might underlie receptor regulation or changes in Ca(2+) handling. Importantly, similar to neurons, perisynaptic glial cells undergo plastic changes induced by altered synaptic activity.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

