Spontaneous lupus-like syndrome in HLA-DQ2 transgenic mice with a mixed genetic background
- PMID: 20142296
- PMCID: PMC2913253
- DOI: 10.1177/0961203309360546
Spontaneous lupus-like syndrome in HLA-DQ2 transgenic mice with a mixed genetic background
Abstract
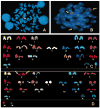
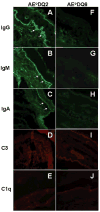
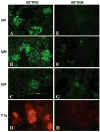
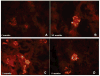
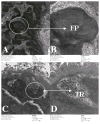
To investigate the role of HLA-DQ2 in the pathogenesis of associated immune disorders, we generated transgenic mice that expressed HLA-DQ2 in the absence of endogenous murine class II molecules (AE(0)DQ2). These AE(0)DQ2 mice with a mixed genetic background spontaneously developed skin lesions on their ears, whereas control AE(0)DQ6 genotype control mice (also with a mixed genetic background) did not. The skin lesions were characterized by deep subepidermal blistering with hydropic degeneration and lymphoid infiltration in the subepidermal area as determined by histopathology. Immunofluorescence analysis revealed thick band-like granular deposition of IgG, IgM, and a thin band of IgA deposition along the basement membrane. AE(0)DQ2 mice also developed significant and progressive hematuria and proteinuria as compared with the AE(0)DQ6 mice (p < 0.05). Histopathology showed immune complex deposits in the glomeruli of AE(0)DQ2 mice. Immunofluorescence analysis showed progressive mesangial and capillary wall deposition of IgA, IgM, IgG and C1q in the kidney. With electron microscopy, the deposits showed a 'fingerprint' substructure; and tubuloreticular structures were identified within endothelial cells. Conversely, these changes were not observed in AE(0)DQ6 mice. Serum anti-double stranded (ds)DNA IgM and IgG levels were also significantly elevated among AE(0)DQ2 mice compared with AE(0)DQ6 mice (p < 0.001). In conclusion, AE(0)DQ2 mice spontaneously develop an autoimmune lupus-like syndrome and are useful model for this disease. It remains to be determined whether genetic admixture played a role in the development of this systemic lupus erythematosus-like syndrome in HLA-DQ2 transgenic mice. Lupus (2010) 19, 815-829.
Figures





References
-
- Martin-Villa JM, Martinez-Laso J, Moreno-Pelayo MA, Castro-Panete MJ, Martinez-Quiles N, Alvarez M, de Juan MD, Gomez-Reino JJ, Arnaiz-Villena A. Differential contribution of HLA-DR, DQ, and TAP2 alleles to systemic lupus erythematosus susceptibility in Spanish patients: role of TAP2*01 alleles in Ro autoantibody production. Ann Rheum Dis. 1998;57:214–9. - PMC - PubMed
-
- Vargas-Alarcon G, Salgado N, Granados J, Gomez-Casado E, Martinez-Laso J, Alcocer-Varela J, Arnaiz-Villena A, Alarcon-Segovia D. Class II allele and haplotype frequencies in Mexican systemic lupus erythematosus patients: the relevance of considering homologous chromosomes in determining susceptibility. Hum Immunol. 2001;62:814–20. - PubMed
-
- Tjernstrom F, Hellmer G, Nived O, Truedsson L, Sturfelt G. Synergetic effect between interleukin-1 receptor antagonist allele (IL1RN*2) and MHC class II (DR17,DQ2) in determining susceptibility to systemic lupus erythematosus. Lupus. 1999;8:103–8. - PubMed
-
- Lambert AP, Gillespie KM, Thomson G, Cordell HJ, Todd JA, Gale EA, Bingley PJ. Absolute risk of childhood-onset type 1 diabetes defined by human leukocyte antigen class II genotype: a population-based study in the United Kingdom. J Clin Endocrinol Metab. 2004;89:4037–43. - PubMed
-
- Segni M, Pani MA, Pasquino AM, Badenhoop K. Familial clustering of juvenile thyroid autoimmunity: higher risk is conferred by human leukocyte antigen DR3-DQ2 and thyroid peroxidase antibody status in fathers. J Clin Endocrinol Metab. 2002;87:3779–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

