Selective serotonin reuptake inhibitors and breast cancer mortality in women receiving tamoxifen: a population based cohort study
- PMID: 20142325
- PMCID: PMC2817754
- DOI: 10.1136/bmj.c693
Selective serotonin reuptake inhibitors and breast cancer mortality in women receiving tamoxifen: a population based cohort study
Abstract
Objective: To characterise whether some selective serotonin reuptake inhibitor (SSRI) antidepressants reduce tamoxifen's effectiveness by inhibiting its bioactivation by cytochrome P450 2D6 (CYP2D6).
Design: Population based cohort study.
Participants: Women living in Ontario aged 66 years or older treated with tamoxifen for breast cancer between 1993 and 2005 who had overlapping treatment with a single SSRI.
Main outcome measures: Risk of death from breast cancer after completion of tamoxifen treatment, as a function of the proportion of time on tamoxifen during which each SSRI had been co-prescribed.
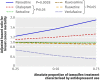
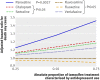
Results: Of 2430 women treated with tamoxifen and a single SSRI, 374 (15.4%) died of breast cancer during follow-up (mean follow-up 2.38 years, SD 2.59). After adjustment for age, duration of tamoxifen treatment, and other potential confounders, absolute increases of 25%, 50%, and 75% in the proportion of time on tamoxifen with overlapping use of paroxetine (an irreversible inhibitor of CYP2D6) were associated with 24%, 54%, and 91% increases in the risk of death from breast cancer, respectively (P<0.05 for each comparison). By contrast, no such risk was seen with other antidepressants. We estimate that use of paroxetine for 41% of tamoxifen treatment (the median overlap in our sample) would result in one additional breast cancer death within five years of cessation of tamoxifen for every 19.7 (95% confidence interval 12.5 to 46.3) patients so treated; the risk with more extensive overlap would be greater.
Conclusion: Paroxetine use during tamoxifen treatment is associated with an increased risk of death from breast cancer, supporting the hypothesis that paroxetine can reduce or abolish the benefit of tamoxifen in women with breast cancer.
Conflict of interest statement
Competing interests: All authors have completed the Unified Competing Interest form at
Figures
Comment in
-
Interaction of serotonin reuptake inhibitors with tamoxifen.BMJ. 2010 Feb 8;340:c783. doi: 10.1136/bmj.c783. BMJ. 2010. PMID: 20142323 No abstract available.
-
ACP Journal Club. Use of paroxetine during tamoxifen therapy for breast cancer was associated with increased breast-cancer and all-cause mortality.Ann Intern Med. 2010 Jun 15;152(12):JC6-13. doi: 10.7326/0003-4819-152-12-201006150-02013. Ann Intern Med. 2010. PMID: 20547895 No abstract available.
References
-
- Anderson BO, Yip CH, Smith RA, Shyyan R, Sener SF, Eniu A, et al. Guideline implementation for breast healthcare in low-income and middle-income countries: overview of the Breast Health Global Initiative Global Summit 2007. Cancer 2008;113(8 Suppl):2221-43. - PubMed
-
- Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;365:1687-717. - PubMed
-
- Desta Z, Ward BA, Soukhova NV, Flockhart DA. Comprehensive evaluation of tamoxifen sequential biotransformation by the human cytochrome P450 system in vitro: prominent roles for CYP3A and CYP2D6. J Pharmacol Exp Ther 2004;310:1062-75. - PubMed
-
- Stearns V, Johnson MD, Rae JM, Morocho A, Novielli A, Bhargava P, et al. Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J Natl Cancer Inst 2003;95:1758-64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical