Localized topological changes of the plasma membrane upon exocytosis visualized by polarized TIRFM
- PMID: 20142424
- PMCID: PMC2819686
- DOI: 10.1083/jcb.200908010
Localized topological changes of the plasma membrane upon exocytosis visualized by polarized TIRFM
Abstract
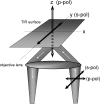
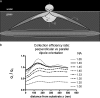
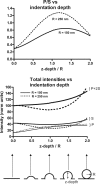
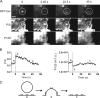
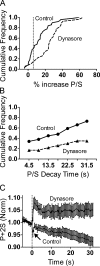
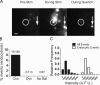
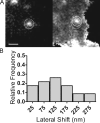
Total internal reflection fluorescence microscopy (TIRFM) images the plasma membrane-cytosol interface and has allowed insights into the behavior of individual secretory granules before and during exocytosis. Much less is known about the dynamics of the other partner in exocytosis, the plasma membrane. In this study, we report the implementation of a TIRFM-based polarization technique to detect rapid submicrometer changes in plasma membrane topology as a result of exocytosis. A theoretical analysis of the technique is presented together with image simulations of predicted topologies of the postfusion granule membrane-plasma membrane complex. Experiments on diI-stained bovine adrenal chromaffin cells using polarized TIRFM demonstrate rapid and varied submicrometer changes in plasma membrane topology at sites of exocytosis that occur immediately upon fusion. We provide direct evidence for a persistent curvature in the exocytotic region that is altered by inhibition of dynamin guanosine triphosphatase activity and is temporally distinct from endocytosis measured by VMAT2-pHluorin.
Figures


References
-
- Artalejo C.R., Elhamdani A., Palfrey H.C. 2002. Sustained stimulation shifts the mechanism of endocytosis from dynamin-1-dependent rapid endocytosis to clathrin- and dynamin-2-mediated slow endocytosis in chromaffin cells. Proc. Natl. Acad. Sci. USA. 99:6358–6363 10.1073/pnas.082658499 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 5T32DA007268/DA/NIDA NIH HHS/United States
- T32 DA007268/DA/NIDA NIH HHS/United States
- P60 DK020572/DK/NIDDK NIH HHS/United States
- DK20572/DK/NIDDK NIH HHS/United States
- R01 MH050712/MH/NIMH NIH HHS/United States
- 1F32GM086169/GM/NIGMS NIH HHS/United States
- P01 DA010154/DA/NIDA NIH HHS/United States
- DA10154/DA/NIDA NIH HHS/United States
- F32 GM086169/GM/NIGMS NIH HHS/United States
- MH50712/MH/NIMH NIH HHS/United States
- R01 NS038129/NS/NINDS NIH HHS/United States
- R01-NS38129/NS/NINDS NIH HHS/United States
- P30 DK020572/DK/NIDDK NIH HHS/United States
- R37 MH050712/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

