Alternative end-joining catalyzes class switch recombination in the absence of both Ku70 and DNA ligase 4
- PMID: 20142431
- PMCID: PMC2822597
- DOI: 10.1084/jem.20092449
Alternative end-joining catalyzes class switch recombination in the absence of both Ku70 and DNA ligase 4
Erratum in
- J Exp Med. 2013 Mar 11;210(3):641
Abstract
The classical nonhomologous end-joining (C-NHEJ) DNA double-strand break (DSB) repair pathway employs the Ku70/80 complex (Ku) for DSB recognition and the XRCC4/DNA ligase 4 (Lig4) complex for ligation. During IgH class switch recombination (CSR) in B lymphocytes, switch (S) region DSBs are joined by C-NHEJ to form junctions either with short microhomologies (MHs; "MH-mediated" joins) or no homologies ("direct" joins). In the absence of XRCC4 or Lig4, substantial CSR occurs via "alternative" end-joining (A-EJ) that generates largely MH-mediated joins. Because upstream C-NHEJ components remain in XRCC4- or Lig4-deficient B cells, residual CSR might be catalyzed by C-NHEJ using a different ligase. To address this, we have assayed for CSR in B cells deficient for Ku70, Ku80, or both Ku70 and Lig4. Ku70- or Ku80-deficient B cells have reduced, but still substantial, CSR. Strikingly, B cells deficient for both Ku plus Lig4 undergo CSR similarly to Ku-deficient B cells, firmly demonstrating that an A-EJ pathway distinct from C-NHEJ can catalyze CSR end-joining. Ku-deficient or Ku- plus Lig4-deficient B cells are also biased toward MH-mediated CSR joins; but, in contrast to XRCC4- or Lig4-deficient B cells, generate substantial numbers of direct CSR joins. Our findings suggest that more than one form of A-EJ can function in CSR.
Figures


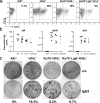
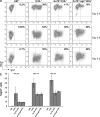
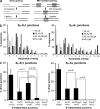
References
-
- Callén E., Jankovic M., Wong N., Zha S., Chen H.T., Difilippantonio S., Di Virgilio M., Heidkamp G., Alt F.W., Nussenzweig A., Nussenzweig M. 2009. Essential role for DNA-PKcs in DNA double-strand break repair and apoptosis in ATM-deficient lymphocytes. Mol. Cell. 34:285–297 10.1016/j.molcel.2009.04.025 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI037526/AI/NIAID NIH HHS/United States
- T32CA09382-26/CA/NCI NIH HHS/United States
- U19 AI031541/AI/NIAID NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- P01 CA092625/CA/NCI NIH HHS/United States
- AI077595/AI/NIAID NIH HHS/United States
- T32 CA009382/CA/NCI NIH HHS/United States
- P01 AI031541/AI/NIAID NIH HHS/United States
- R01 AI077595/AI/NIAID NIH HHS/United States
- R37 AI077595/AI/NIAID NIH HHS/United States
- CA092625/CA/NCI NIH HHS/United States
- AI037526/AI/NIAID NIH HHS/United States
- R37 AI037526/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

