Th17 and Th1 T-cell responses in giant cell arteritis
- PMID: 20142449
- PMCID: PMC2837465
- DOI: 10.1161/CIRCULATIONAHA.109.872903
Th17 and Th1 T-cell responses in giant cell arteritis
Abstract
Background: In giant cell arteritis (GCA), vasculitic damage of the aorta and its branches is combined with a syndrome of intense systemic inflammation. Therapeutically, glucocorticoids remain the gold standard because they promptly and effectively suppress acute manifestations; however, they fail to eradicate vessel wall infiltrates. The effects of glucocorticoids on the systemic and vascular components of GCA are not understood.
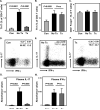
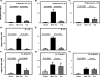
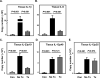
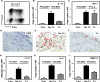
Methods and results: The immunoprofile of untreated and glucocorticoid-treated GCA was examined in peripheral blood and temporal artery biopsies with protein quantification assays, flow cytometry, quantitative real-time polymerase chain reaction, and immunohistochemistry. Plasma interferon-gamma and interleukin (IL)-17 and frequencies of interferon-gamma-producing and IL-17-producing T cells were markedly elevated before therapy. Glucocorticoid treatment suppressed the Th17 but not the Th1 arm in the blood and the vascular lesions. Analysis of monocytes/macrophages in the circulation and in temporal arteries revealed glucocorticoid-mediated suppression of Th17-promoting cytokines (IL-1beta, IL-6, and IL-23) but sparing of Th1-promoting cytokines (IL-12). In human artery-severe combined immunodeficiency mouse chimeras, in which patient-derived T cells cause inflammation of engrafted human temporal arteries, glucocorticoids were similarly selective in inhibiting Th17 cells and leaving Th1 cells unaffected.
Conclusions: Two pathogenic pathways mediated by Th17 and Th1 cells contribute to the systemic and vascular manifestations of GCA. IL-17-producing Th17 cells are sensitive to glucocorticoid-mediated suppression, but interferon-gamma-producing Th1 responses persist in treated patients. Targeting steroid-resistant Th1 responses will be necessary to resolve chronic smoldering vasculitis. Monitoring Th17 and Th1 frequencies can aid in assessing disease activity in GCA.
Figures

References
-
- Weyand CM, Goronzy JJ. Giant-cell arteritis and polymyalgia rheumatica. Ann Intern Med. 2003;139:505–515. - PubMed
-
- Evans JM, O'Fallon WM, Hunder GG. Increased incidence of aortic aneurysm and dissection in giant cell (temporal) arteritis. A population-based study. Ann Intern Med. 1995;122:502–507. - PubMed
-
- Hoffman GS, Cid MC, Rendt-Zagar KE, Merkel PA, Weyand CM, Stone JH, Salvarani C, Xu W, Visvanathan S, Rahman MU. Infliximab for maintenance of glucocorticosteroid-induced remission of giant cell arteritis: a randomized trial. Ann Intern Med. 2007;146:621–630. - PubMed
-
- Salvarani C, Macchioni P, Manzini C, Paolazzi G, Trotta A, Manganelli P, Cimmino M, Gerli R, Catanoso MG, Boiardi L, Cantini F, Klersy C, Hunder GG. Infliximab plus prednisone or placebo plus prednisone for the initial treatment of polymyalgia rheumatica: a randomized trial. Ann Intern Med. 2007;146:631–639. - PubMed
-
- Mahr AD, Jover JA, Spiera RF, Hernandez-Garcia C, Fernandez-Gutierrez B, Lavalley MP, Merkel PA. Adjunctive methotrexate for treatment of giant cell arteritis: an individual patient data meta-analysis. Arthritis Rheum. 2007;56:2789–2797. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI044142/AI/NIAID NIH HHS/United States
- P01 HL058000/HL/NHLBI NIH HHS/United States
- R01 HL117913/HL/NHLBI NIH HHS/United States
- R01 AG 015043/AG/NIA NIH HHS/United States
- U19 AI 57266/AI/NIAID NIH HHS/United States
- UL1 RR025744/RR/NCRR NIH HHS/United States
- ULI RR025744/RR/NCRR NIH HHS/United States
- R01 EY011916/EY/NEI NIH HHS/United States
- U19 AI057266/AI/NIAID NIH HHS/United States
- R01 AR042527/AR/NIAMS NIH HHS/United States
- R01 AR041974/AR/NIAMS NIH HHS/United States
- R01 EY 11916/EY/NEI NIH HHS/United States
- P01 HL 058000/HL/NHLBI NIH HHS/United States
- R01 AG015043/AG/NIA NIH HHS/United States
- R01 AR 042527/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

