Targeted metabolic labeling of yeast N-glycans with unnatural sugars
- PMID: 20142501
- PMCID: PMC2840165
- DOI: 10.1073/pnas.0911247107
Targeted metabolic labeling of yeast N-glycans with unnatural sugars
Abstract
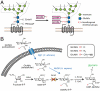
Metabolic labeling of glycans with synthetic sugar analogs has emerged as an attractive means for introducing nonnatural chemical functionality into glycoproteins. However, the complexities of glycan biosynthesis prevent the installation of nonnatural moieties at defined, predictable locations within glycoproteins at high levels of incorporation. Here, we demonstrate that the conserved N-acetyglucosamine (GlcNAc) residues within chitobiose cores of N-glycans in the model organism Saccharomyces cerevisiae can be specifically targeted for metabolic replacement by unnatural sugars. We introduced an exogenous GlcNAc salvage pathway into yeast, allowing cells to metabolize GlcNAc provided as a supplement to the culture medium. We then rendered the yeast auxotrophic for production of the donor nucleotide-sugar uridine-diphosphate-GlcNAc (UDP-GlcNAc) by deletion of the essential gene GNA1. We demonstrate that gna1Delta strains require a GlcNAc supplement and that expression plasmids containing both exogenous components of the salvage pathway, GlcNAc transporter NGT1 from Candida albicans and GlcNAc kinase NAGK from Homo sapiens, are required for rescue in this context. Further, we show that cells successfully incorporate synthetic GlcNAc analogs N-azidoacetyglucosamine (GlcNAz) and N-(4-pentynoyl)-glucosamine (GlcNAl) into cell-surface glycans and secreted glycoproteins. To verify incorporation of the nonnatural sugars at N-glycan core positions, endoglycosidase H (endoH)-digested peptides from a purified secretory glycoprotein, Ygp1, were analyzed by mass spectrometry. Multiple Ygp1 N-glycosylation sites bearing GlcNAc, isotopically labeled GlcNAc, or GlcNAz were identified; these modifications were dependent on the supplement added to the culture medium. This system enables the production of glycoproteins that are functionalized for specific chemical modifications at their glycosylation sites.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
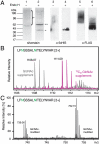
 . Ygp1 was treated with endoH, trypsinized, and subjected to ESI-FTICR MS analysis. Masses for a representative glycopeptide, spanning Leu 98-Arg 115 and glycosylated at only one of two potential sites (indicated in green), are shown. Relative intensities of the GlcNAc- and
. Ygp1 was treated with endoH, trypsinized, and subjected to ESI-FTICR MS analysis. Masses for a representative glycopeptide, spanning Leu 98-Arg 115 and glycosylated at only one of two potential sites (indicated in green), are shown. Relative intensities of the GlcNAc- and  -modified peptides have been normalized to each other. (C) Ygp1 was expressed in culture medium supplemented with GlcNAz and subjected to ESI-FTICR MS analysis. Masses corresponding to the same glycopeptide from (B) are shown.
-modified peptides have been normalized to each other. (C) Ygp1 was expressed in culture medium supplemented with GlcNAz and subjected to ESI-FTICR MS analysis. Masses corresponding to the same glycopeptide from (B) are shown.References
-
- Link AJ, Mock ML, Tirrell DA. Non-canonical amino acids in protein engineering. Curr Opin Biotechnol. 2003;14:603–609. - PubMed
-
- Wang L, Schultz PG. Expanding the genetic code. Angew Chem Int Ed Engl. 2004;44:34–66. - PubMed
-
- Campbell CT, Sampathkumar SG, Yarema KJ. Metabolic oligosaccharide engineering: Perspectives, applications, and future directions. Mol Biosyst. 2007;3:187–194. - PubMed
-
- Nandi A, et al. Global identification of O-GlcNAc-modified proteins. Anal Chem. 2006;78:452–458. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

