Development of choroidal neovascularization in rats with advanced intense cyclic light-induced retinal degeneration
- PMID: 20142545
- PMCID: PMC2820133
- DOI: 10.1001/archophthalmol.2009.395
Development of choroidal neovascularization in rats with advanced intense cyclic light-induced retinal degeneration
Abstract
Objectives: To study the progressive changes of intense cyclic light-induced retinal degeneration and to determine whether it results in choroidal neovascularization (CNV).
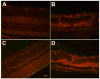
Methods: Albino rats were exposed to 12 hours of 3000-lux cyclic light for 1, 3, or 6 months. Fundus examination, fundus photography, fluorescein and indocyanine green angiography, and optical coherence tomography were performed prior to euthanization. Light-exposed animals were euthanized after 1, 3, or 6 months for histopathological evaluation. Retinas were examined for the presence of 4-hydroxy-2-nonenal- and nitrotyrosine-modified proteins by immunofluorescence staining.
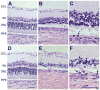
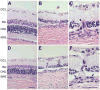
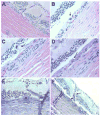
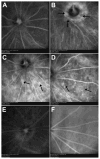
Results: Long-term intense cyclic light exposure resulted in retinal degeneration with loss of the outer segments of photoreceptors and approximately two-thirds of the outer nuclear layer as well as development of subretinal pigment epithelium neovascularization after 1 month. Almost the entire outer nuclear layer was absent with the presence of CNV, which penetrated the Bruch membrane and extended into the outer retina after 3 months. Absence of the outer nuclear layer, multiple foci of CNV, retinal pigment epithelial fibrous metaplasia, and connective tissue bands containing blood vessels extending into the retina were observed after 6 months. All intense light-exposed animals showed an increased presence of 4-hydroxy-2-nonenal and nitrotyrosine staining. Optical coherence tomographic and angiographic studies confirmed retinal thinning and leakiness of the newly formed blood vessels.
Conclusions: Our results suggest that albino rats develop progressive stages of retinal degeneration and CNV after long-term intense cyclic light exposure, allowing the detailed study of the pathogenesis and treatment of age-related macular degeneration.
Clinical relevance: The ability to study the progressive pathogenesis of age-related macular degeneration and CNV will provide detailed knowledge about the disease and aid in the development of target-specific therapy.
Figures


Comment in
-
Intense cyclic light-induced retinal degeneration in rats.Arch Ophthalmol. 2010 Feb;128(2):244-5. doi: 10.1001/archophthalmol.2009.399. Arch Ophthalmol. 2010. PMID: 20142550 Free PMC article. No abstract available.
References
-
- Ambati J, Ambati BK, Yoo SH, Ianchulev S, Adamis AP. Age-related macular degeneration: etiology, pathogenesis, and therapeutic strategies. Surv Ophthalmol. 2003;48:257–293. - PubMed
-
- Jager RD, Mieler WF, Miller JW. Age-Related Macular Degeneration. N Engl J Med. 2008;358:2606–2617. - PubMed
-
- Jager RD, Mieler WF, Miller JW. Age-related macular degeneration. N Engl J Med. 2008;358:2606–2617. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

