Acute selenium toxicity associated with a dietary supplement
- PMID: 20142570
- PMCID: PMC3225252
- DOI: 10.1001/archinternmed.2009.495
Acute selenium toxicity associated with a dietary supplement
Abstract
Background: Selenium is an element necessary for normal cellular function, but it can have toxic effects at high doses. We investigated an outbreak of acute selenium poisoning.
Methods: A case was defined as the onset of symptoms of selenium toxicity in a person within 2 weeks after ingesting a dietary supplement manufactured by "Company A," purchased after January 1, 2008. We conducted case finding, administered initial and 90-day follow-up questionnaires to affected persons, and obtained laboratory data where available.
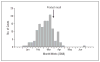
Results: The source of the outbreak was identified as a liquid dietary supplement that contained 200 times the labeled concentration of selenium. Of 201 cases identified in 10 states, 1 person was hospitalized. The median estimated dose of selenium consumed was 41 749 microg/d (recommended dietary allowance is 55 microg/d). Frequently reported symptoms included diarrhea (78%), fatigue (75%), hair loss (72%), joint pain (70%), nail discoloration or brittleness (61%), and nausea (58%). Symptoms persisting 90 days or longer included fingernail discoloration and loss (52%), fatigue (35%), and hair loss (29%). The mean initial serum selenium concentration of 8 patients was 751 microg/L (reference range, < or =125 microg/L). The mean initial urine selenium concentration of 7 patients was 166 microg/24 h (reference range, < or =55 microg/24 h).
Conclusions: Toxic concentrations of selenium in a liquid dietary supplement resulted in a widespread outbreak. Had the manufacturers been held to standards used in the pharmaceutical industry, it may have been prevented.
Figures
Comment in
-
The dietary supplement health and education act: time for a reassessment: comment on "acute selenium toxicity associated with a dietary supplement".Arch Intern Med. 2010 Feb 8;170(3):261-3. doi: 10.1001/archinternmed.2009.480. Arch Intern Med. 2010. PMID: 20142571 No abstract available.
References
-
- Agency for Toxic Substances and Disease Registry (ATSDR) Toxicologic Profile for Selenium. Atlanta, GA: US Department of Health and Human Services, Public Health Service; 2003.
-
- Institute of Medicine (IOM) Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington, DC: National Academy Press; 2000. Selenium; pp. 284–324.
-
- Levander OA. Scientific rationale for the 1989 recommended dietary allowance for selenium. J Am Diet Assoc. 1991;91(12):1572–1576. - PubMed
-
- Nuttall KL. Evaluating selenium poisoning. Ann Clin Lab Sci. 2006;36(4):409–420. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical