Phase II, randomized, open-label study of pegfilgrastim-supported VDC/IE chemotherapy in pediatric sarcoma patients
- PMID: 20142595
- PMCID: PMC2834494
- DOI: 10.1200/JCO.2009.24.8872
Phase II, randomized, open-label study of pegfilgrastim-supported VDC/IE chemotherapy in pediatric sarcoma patients
Abstract
This multicenter, randomized, open-label study evaluated the efficacy, safety, and pharmacokinetics of a single subcutaneous pegfilgrastim injection with daily subcutaneous filgrastim administration in pediatric patients receiving myelosuppressive chemotherapy for sarcoma. PATIENTS AND METHODS Forty-four patients with previously untreated, biopsy-proven sarcoma stratified into three age groups (0-5, 6-11, and 12-21 years) were randomly assigned in a 6:1 randomization ratio to receive a single pegfilgrastim dose of 100 microg/kg (n = 38) or daily filgrastim doses of 5 microg/kg (n = 6) after chemotherapy (cycles 1 and 3: vincristine-doxorubicin-cyclophosphamide; cycles 2 and 4: ifosfamide-etoposide). The duration of grade 4 neutropenia, time to neutrophil recovery, incidence of febrile neutropenia, and adverse events were recorded. Results Pegfilgrastim and filgrastim were similar for all efficacy and safety end points, and their pharmacokinetic profiles were consistent with those in adults. Younger children experienced more protracted neutropenia and had higher median pegfilgrastim exposure than older children. CONCLUSION A single dose of pegfilgrastim at 100 microg/kg administered once per chemotherapy cycle is comparable to daily injections of filgrastim at 5 microg/kg for pediatric sarcoma patients receiving myelosuppressive chemotherapy.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
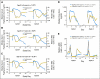
Similar articles
-
Comparable efficacy and safety profiles of once-per-cycle pegfilgrastim and daily injection filgrastim in chemotherapy-induced neutropenia: a multicenter dose-finding study in women with breast cancer.Ann Oncol. 2002 Jun;13(6):903-9. doi: 10.1093/annonc/mdf130. Ann Oncol. 2002. PMID: 12123336 Clinical Trial.
-
Randomized, multicenter, open-label study of pegfilgrastim compared with daily filgrastim after chemotherapy for lymphoma.J Clin Oncol. 2003 Feb 1;21(3):514-9. doi: 10.1200/JCO.2003.03.040. J Clin Oncol. 2003. PMID: 12560443 Clinical Trial.
-
Randomized trial and pharmacokinetic study of pegfilgrastim versus filgrastim after dose-intensive chemotherapy in young adults and children with sarcomas.Clin Cancer Res. 2009 Dec 1;15(23):7361-7. doi: 10.1158/1078-0432.CCR-09-0761. Epub 2009 Nov 17. Clin Cancer Res. 2009. PMID: 19920107 Free PMC article. Clinical Trial.
-
Pegfilgrastim administered once per cycle reduces incidence of chemotherapy-induced neutropenia.Drugs. 2002;62 Suppl 1:89-98. doi: 10.2165/00003495-200262001-00007. Drugs. 2002. PMID: 12479597 Review.
-
Once-per-cycle pegfilgrastim (Neulasta) for the management of chemotherapy-induced neutropenia.Semin Oncol. 2003 Aug;30(4 Suppl 13):24-30. doi: 10.1016/s0093-7754(03)00314-2. Semin Oncol. 2003. PMID: 14508717 Review.
Cited by
-
Prophylactic long-acting granulocyte-colony stimulating factors (G-CSF) in gynecologic malignancies: an oncologic expert statement.Wien Med Wochenschr. 2015 Oct;165(19-20):387-94. doi: 10.1007/s10354-015-0392-3. Epub 2015 Oct 15. Wien Med Wochenschr. 2015. PMID: 26471371 Review.
-
Mecapegfilgrastim for prophylaxis of febrile neutropenia in children and adolescents with rhabdomyosarcoma or Ewing sarcoma: a prospective, single-arm, pilot study.BMC Cancer. 2024 Aug 15;24(1):1013. doi: 10.1186/s12885-024-12766-w. BMC Cancer. 2024. PMID: 39148050 Free PMC article. Clinical Trial.
-
[Pharmacokinetics and pharmacodynamics of pegylated recombinant human granulocyte colony-stimulating factor in children with acute lymphoblastic leukemia: a prospective control trial].Zhongguo Dang Dai Er Ke Za Zhi. 2020 Nov;22(11):1172-1177. doi: 10.7499/j.issn.1008-8830.2005048. Zhongguo Dang Dai Er Ke Za Zhi. 2020. PMID: 33172550 Free PMC article. Chinese.
-
Quantification of Radiation Injury on Neutropenia and the Link between Absolute Neutrophil Count Time Course and Overall Survival in Nonhuman Primates Treated with G-CSF.Pharm Res. 2020 May 21;37(6):102. doi: 10.1007/s11095-020-02839-3. Pharm Res. 2020. PMID: 32440783 Free PMC article.
-
A pharmacokinetic study of lipegfilgrastim in children with Ewing family of tumors or rhabdomyosarcoma.Cancer Chemother Pharmacol. 2017 Jan;79(1):155-164. doi: 10.1007/s00280-016-3216-2. Epub 2016 Dec 16. Cancer Chemother Pharmacol. 2017. PMID: 27986986 Free PMC article. Clinical Trial.
References
-
- Bacci G, Picci P, Avella M, et al. The importance of dose-intensity in neoadjuvant chemotherapy of osteosarcoma: A retrospective analysis of high-dose methotrexate, cisplatinum and adriamycin used preoperatively. J Chemother. 1990;2:127–135. - PubMed
-
- Baker KS, Anderson JR, Link MP, et al. Benefit of intensified therapy for patients with local or regional embryonal rhabdomyosarcoma: Results from the Intergroup Rhabdomyosarcoma Study IV. J Clin Oncol. 2000;18:2427–2434. - PubMed
-
- Smith MA, Ungerleider RS, Horowitz ME, et al. Influence of doxorubicin dose intensity on response and outcome for patients with osteogenic sarcoma and Ewing's sarcoma. J Natl Cancer Inst. 1991;83:1460–1470. - PubMed
-
- Grier HE, Krailo MD, Tarbell NJ, et al. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing's sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med. 2003;348:694–701. - PubMed
-
- Ferrari S, Smeland S, Mercuri M, et al. Neoadjuvant chemotherapy with high-dose Ifosfamide, high-dose methotrexate, cisplatin, and doxorubicin for patients with localized osteosarcoma of the extremity: A joint study by the Italian and Scandinavian Sarcoma Groups. J Clin Oncol. 2005;23:8845–8852. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

