Cellular histone modification patterns predict prognosis and treatment response in resectable pancreatic adenocarcinoma: results from RTOG 9704
- PMID: 20142597
- PMCID: PMC2834495
- DOI: 10.1200/JCO.2009.24.5639
Cellular histone modification patterns predict prognosis and treatment response in resectable pancreatic adenocarcinoma: results from RTOG 9704
Abstract
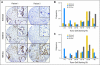
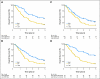
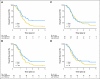
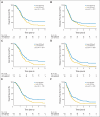
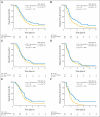
PURPOSE Differences in cellular levels of histone modifications have predicted clinical outcome in certain cancers. Here, we studied the prognostic and predictive value of three histone modifications in pancreatic adenocarcinoma. METHODS Tissue microarrays (TMAs) from two pancreatic adenocarcinoma cohorts were examined, including those from a 195-patient cohort from Radiation Therapy Oncology Group trial RTOG 9704, a multicenter, phase III, randomized treatment trial comparing adjuvant gemcitabine with fluorouracil and a 140-patient cohort of patients with stage I or II cancer from University of California, Los Angeles Medical Center. Immunohistochemistry was performed for histone H3 lysine 4 dimethylation (H3K4me2), histone H3 lysine 9 dimethylation (H3K9me2), and histone H3 lysine 18 acetylation (H3K18ac). Positive tumor cell staining for each histone modification was used to classify patients into low- and high-staining groups, which were related to clinicopathologic parameters and clinical outcome measures. Results Low cellular levels of H3K4me2, H3K9me2, or H3K18ac were each significant and independent predictors of poor survival in univariate and multivariate models, and combined low levels of H3K4me2 and/or H3K18ac were the most significant predictor of overall survival (hazard ratio, 2.93; 95% CI, 1.78 to 4.82) in the University of California, Los Angeles cohort. In subgroup analyses, histone levels were predictive of survival specifically for those patients with node-negative cancer or for those patients receiving adjuvant fluorouracil, but not gemcitabine, in RTOG 9704. CONCLUSION Cellular levels of histone modifications define previously unrecognized subsets of patients with pancreatic adenocarcinoma with distinct epigenetic phenotypes and clinical outcomes and represent prognostic and predictive biomarkers that could inform clinical decisions, including the use of fluorouracil chemotherapy.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
References
-
- Hezel AF, Kimmelman AC, Stanger BZ, et al. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 2006;20:1218–1249. - PubMed
-
- Bernstein BE, Meissner A, Lander ES. The mammalian epigenome. Cell. 2007;128:669–681. - PubMed
-
- Ting AH, McGarvey KM, Baylin SB. The cancer epigenome: Components and functional correlates. Genes Dev. 2006;20:3215–3231. - PubMed
-
- Esteller M. Cancer epigenomics: DNA methylomes and histone-modification maps. Nat Rev Genet. 2007;8:286–298. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

