Long-term outcomes for infants with very low risk Wilms tumor treated with surgery alone in National Wilms Tumor Study-5
- PMID: 20142733
- PMCID: PMC2836016
- DOI: 10.1097/SLA.0b013e3181c0e5d7
Long-term outcomes for infants with very low risk Wilms tumor treated with surgery alone in National Wilms Tumor Study-5
Abstract
Objective: To determine the event-free survival (EFS) and overall survival (OS) of children with very low risk Wilms tumor (VLRWT) treated with surgery only.
Background: Previous studies suggested that postoperative chemotherapy had not improved the prognosis of children with VLRWT. A total of 77 children <24 months of age with small (<550 g) Stage I favorable histology Wilms tumors were treated with surgery only. This study was closed based on stopping rules to ensure that the 2-year EFS was > or =90%.
Methods: A total of 77 children were assessed for EFS and OS. Of these patients, 21 enrolled at the time of closure were recalled, treated with dactinomycin and vincristine (regimen EE4A), and censored for analysis thereafter. About 111 children subsequently treated with EE4A were available for comparison.
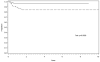
Results: Median follow-up of surviving patients was 8.2 years for surgery only (range, 1.9-11.8 years) and 5.2 years for the EE4A group (range, 1.6-8.9 years). The estimated 5-year EFS for surgery only was 84% (95% confidence interval [CI]: 73%, 91%); for the EE4A patients it was 97% (95% CI: 92%, 99%, P = 0.002). One death was observed in each treatment group. The estimated 5-year OS was 98% (95% CI: 87%, 99%) for surgery only and 99% (95% CI: 94%, 99%) for EE4A (P = 0.70).
Conclusion: The surgery-only EFS was lower than anticipated but, coupled with a much higher than anticipated salvage rate of the chemotherapy naive patients whose disease recurred, led to an observed long-term OS equivalent to that seen with 2-drug chemotherapy. This approach to the treatment of patients with VLRWT eliminates the toxic side-effects of chemotherapy for a large majority of patients. A follow-up study is underway to confirm these findings.
Figures
References
-
- Green DM, Breslow N, Beckwith JB, et al. Treatment with nephrectomy only for small, stage I/Favorable histology Wilms' tumor: A report from the National Wilms' Tumor Study Group. J Clin Oncol. 2001;19(17):3719–3724. - PubMed
-
- Green D, Cotton C, Malogolowkin M, et al. Treatment of Wilms tumor relapsing after initial treatment with Vincristine and Actinomycin D: A Report from the National Wilms Tumor Study Group. Pediatr Blood Cancer. 2007;48:493–499. - PubMed
-
- Malogolowkin MH, Feusner J, Steele DA, et al. Carboplatin (CBDCA)/Etoposide (VP-16) for the treatment of children with high-risk (HR) or recurrent Wilms' tumor (RWT) ASCO. 1994;13:424.
-
- Farber S, D'Angio GJ, Evans A, Mitus A. Clinical studies of actinomycin D with special reference to Wilms' Tumor in children. Ann NY Acad Sci. 1960;89:421–425. - PubMed
-
- Farber S. Chemotherapy in the treatment of leukemia and Wilms' Tumor. JAMA. 1966;198(8):826–836. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical