Sensors of the innate immune system: their link to rheumatic diseases
- PMID: 20142813
- PMCID: PMC4437225
- DOI: 10.1038/nrrheum.2009.278
Sensors of the innate immune system: their link to rheumatic diseases
Abstract
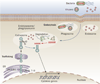
Evidence strongly suggests that excessive or protracted signaling, or both, by cell-surface or intracellular innate immune receptors is central to the pathogenesis of most autoimmune and autoinflammatory rheumatic diseases. The initiation of aberrant innate and adaptive immune responses in autoimmune diseases can be triggered by microbes and, at times, by endogenous molecules--particularly nucleic acids and related immune complexes--under sterile conditions. By contrast, most autoinflammatory syndromes are generally dependent on germline or de novo gene mutations that cause or facilitate inflammasome assembly. The consequent production of proinflammatory cytokines, principally interferon-alpha/beta and tumor necrosis factor in autoimmune diseases, and interleukin-1beta in autoinflammatory diseases, leads to the creation of autoamplification feedback loops and chronicity of these syndromes. These findings have resulted in a critical reappraisal of pathogenetic mechanisms, and provide a basis for the development of novel diagnostic and therapeutic modalities for these diseases.
Figures




References
-
- Baccala R, et al. Sensors of the innate immune system: their mode of action. Nat. Rev. Rheumatol. 2009;5:448–456. - PubMed
-
- Ishii KJ, Akira S. TLR ignores methylated RNA? Immunity. 2005;23:111–113. - PubMed
-
- Schlee M, et al. Approaching the RNA ligand for RIG-I? Immunol. Rev. 2009;227:66–74. - PubMed
-
- Bave U, Vallin H, Alm GV, Ronnblom L. Activation of natural interferon-alpha producing cells by apoptotic U937 cells combined with Iupus IgG and its regulation by cytokines. J. Autoimmun. 2001;17:71–80. - PubMed
-
- Leadbetter EA, et al. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:603–607. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

