ASPM-associated stem cell proliferation is involved in malignant progression of gliomas and constitutes an attractive therapeutic target
- PMID: 20142996
- PMCID: PMC2817685
- DOI: 10.1186/1475-2867-10-1
ASPM-associated stem cell proliferation is involved in malignant progression of gliomas and constitutes an attractive therapeutic target
Erratum in
-
Correction: ASPM-associated stem cell proliferation is involved in malignant progression of gliomas and constitutes an attractive therapeutic target.Cancer Cell Int. 2011 Apr 15;11:10. doi: 10.1186/1475-2867-11-10. Cancer Cell Int. 2011. PMID: 21496261 Free PMC article. No abstract available.
Abstract
Background: ASPM (Abnormal Spindle-like Microcephaly associated) over-expression was recently implicated in the development of malignant gliomas.
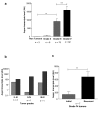
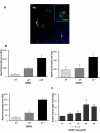
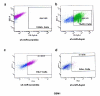
Results: To better characterize the involvement of ASPM in gliomas, we investigated the mRNA expression in 175 samples, including 8 WHO Grade II, 75 WHO Grade III and 92 WHO Grade IV tumors. Aspm expression was strongly correlated with tumor grade and increased at recurrence when compared to the initial lesion, whatever the initial grade of the primary tumor. ASPM expression also increased over serial passages in gliomaspheres in vitro and in mouse xenografts in vivo. Lentivirus-mediated shRNA silencing of ASPM resulted in dramatic proliferation arrest and cell death in two different gliomasphere models.
Conclusion: These data suggest that ASPM is involved in the malignant progression of gliomas, possibly through expansion of a cancer stem cell compartment, and is an attractive therapeutic target in glioblastoma multiforme.
Figures

References
-
- Kouprina N, Pavlicek A, Collins NK, Nakano M, Noskov VN, Ohzeki J, Mochida GH, Risinger JI, Goldsmith P, Gunsior M. The microcephaly ASPM gene is expressed in proliferating tissues and encodes for a mitotic spindle protein. Hum Mol Genet. 2005;14(15):2155–2165. doi: 10.1093/hmg/ddi220. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

