Liposomal simvastatin attenuates neointimal hyperplasia in rats
- PMID: 20143196
- PMCID: PMC2844514
- DOI: 10.1208/s12248-010-9173-5
Liposomal simvastatin attenuates neointimal hyperplasia in rats
Abstract
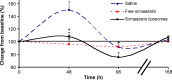
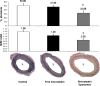
Monocytes, macrophages, and inflammation play a key role in the process of neointimal proliferation and restenosis. The present study evaluated whether systemic and transient depletion of monocytes could be obtained by a single intravenous (IV) injection of simvastatin liposomes, for the inhibition of neointima formation. Balloon-injured carotid artery rats (n = 30) were randomly assigned to treatment groups of free simvastatin, simvastatin in liposomes (3 mg/kg), and saline (control). Stenosis and neointima to media ratio (N/M) were determined 14 days following single IV injection at the time of injury by morphometric analysis. Depletion of circulating monocytes was determined by flow cytometry analyzes of blood specimens. Inhibition of RAW264.7, J774, and THP-1 proliferation by simvastatin-loaded liposomes and free simvastatin was determined by the 3-(4, 5-dimethylthiazolyl-2)-2, 5- diphenyltetrazolium bromide assay. Simvastatin liposomes were successfully formulated and were found to be 1.5-2 times more potent than the free drug in suppressing the proliferation of monocytes/macrophages in cell cultures of RAW 264.7, J774, and THP-1. IV injection of liposomal simvastatin to carotid-injured rats (3 mg/kg, n = 4) resulted in a transient depletion of circulating monocytes, significantly more prolonged than that observed following treatment with free simvastatin. Administration to balloon-injured rats suppressed neointimal growth. N/M at 14 days was 1.56 +/- 0.16 and 0.90 +/- 0.12, control and simvastatin liposomes, respectively. One single systemic administration of liposomal simvastatin at the time of injury significantly suppresses neointimal formation in the rat model of restenosis, mediated via a partial and transient depletion of circulating monocytes.
Figures
Similar articles
-
Intraluminal Delivery of Simvastatin Attenuates Intimal Hyperplasia After Arterial Injury.Vasc Endovascular Surg. 2019 Jul;53(5):379-386. doi: 10.1177/1538574419833224. Epub 2019 Apr 14. Vasc Endovascular Surg. 2019. PMID: 30982448
-
Effects of hydroxymethylglutaryl coenzyme A reductase inhibitor simvastatin on smooth muscle cell proliferation in vitro and neointimal formation in vivo after vascular injury.J Am Coll Cardiol. 2000 Jan;35(1):214-21. doi: 10.1016/s0735-1097(99)00526-4. J Am Coll Cardiol. 2000. PMID: 10636283
-
Nanosuspensions of alendronate with gallium or gadolinium attenuate neointimal hyperplasia in rats.J Control Release. 2007 Feb 26;117(3):322-32. doi: 10.1016/j.jconrel.2006.10.030. Epub 2006 Nov 11. J Control Release. 2007. PMID: 17234295
-
Systemic depletion of macrophages by liposomal bisphosphonates reduces neointimal formation following balloon-injury in the rat carotid artery.J Cardiovasc Pharmacol. 2003 Nov;42(5):671-9. doi: 10.1097/00005344-200311000-00014. J Cardiovasc Pharmacol. 2003. PMID: 14576517
-
Sirolimus-loaded stealth colloidal systems attenuate neointimal hyperplasia after balloon injury: a comparison of phospholipid micelles and liposomes.Int J Pharm. 2013 Oct 15;455(1-2):320-30. doi: 10.1016/j.ijpharm.2013.07.003. Epub 2013 Jul 16. Int J Pharm. 2013. PMID: 23867987
Cited by
-
Bioengineering Strategies for Treating Neointimal Hyperplasia in Peripheral Vasculature: Innovations and Challenges.Adv Healthc Mater. 2025 Mar;14(7):e2401056. doi: 10.1002/adhm.202401056. Epub 2025 Jan 31. Adv Healthc Mater. 2025. PMID: 39888207 Free PMC article. Review.
-
Monocyte-mediated drug delivery systems for the treatment of cardiovascular diseases.Drug Deliv Transl Res. 2018 Aug;8(4):868-882. doi: 10.1007/s13346-017-0431-2. Drug Deliv Transl Res. 2018. PMID: 29058205 Review.
-
Extracellular Vesicles in Cardiovascular Diseases: Alternative Biomarker Sources, Therapeutic Agents, and Drug Delivery Carriers.Int J Mol Sci. 2019 Jul 3;20(13):3272. doi: 10.3390/ijms20133272. Int J Mol Sci. 2019. PMID: 31277271 Free PMC article. Review.
-
Charge effect of a liposomal delivery system encapsulating simvastatin to treat experimental ischemic stroke in rats.Int J Nanomedicine. 2016 Jun 29;11:3035-48. doi: 10.2147/IJN.S107292. eCollection 2016. Int J Nanomedicine. 2016. PMID: 27418824 Free PMC article.
-
Immunoliposomes with Simvastatin as a Potential Therapeutic in Treatment of Breast Cancer Cells Overexpressing HER2-An In Vitro Study.Cancers (Basel). 2018 Nov 1;10(11):418. doi: 10.3390/cancers10110418. Cancers (Basel). 2018. PMID: 30388834 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

