Dopamine system dysregulation by the ventral subiculum as the common pathophysiological basis for schizophrenia psychosis, psychostimulant abuse, and stress
- PMID: 20143199
- PMCID: PMC2932888
- DOI: 10.1007/s12640-010-9154-6
Dopamine system dysregulation by the ventral subiculum as the common pathophysiological basis for schizophrenia psychosis, psychostimulant abuse, and stress
Abstract
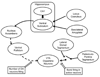
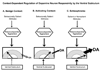
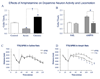
The dopamine system is under multiple forms of regulation, and in turn provides effective modulation of system responses. Dopamine neurons are known to exist in several states of activity. The population activity, or the proportion of dopamine neurons firing spontaneously, is controlled by the ventral subiculum of the hippocampus. In contrast, burst firing, which is proposed to be the behaviorally salient output of the dopamine system, is driven by the brainstem pedunculopontine tegmentum (PPTg). When an animal is exposed to a behaviorally salient stimulus, the PPTg elicits a burst of action potentials in the dopamine neurons. However, this bursting only occurs in the portion of the dopamine neuron population that is firing spontaneously. This proportion is regulated by the ventral subiculum. Therefore, the ventral subiculum provides the gain, or the amplification factor, for the behaviorally salient stimulus. The ventral subiculum itself is proposed to carry information related to the environmental context. Thus, the ventral subiculum will adjust the responsivity of the dopamine system based on the needs of the organism and the characteristics of the environment. However, this finely tuned system can be disrupted in disease states. In schizophrenia, a disruption of interneuronal regulation of the ventral subiculum is proposed to lead to an overdrive of the dopamine system, rendering the system in a constant hypervigilant state. Moreover, amphetamine sensitization and stressors also appear to cause an abnormal dopaminergic drive. Such an interaction could underlie the risk factors of drug abuse and stress in the precipitation of a psychotic event. On the other hand, this could point to the ventral subiculum as an effective site of therapeutic intervention in the treatment or even the prevention of schizophrenia.
Figures
References
-
- Abercrombie ED, Keefe KA, DiFrischia DS, Zigmond MJ. Differential effect of stress on in vivo dopamine release in striatum, nucleus accumbens, and medial frontal cortex. J Neurochem. 1989;52:1655–1658. - PubMed
-
- Amaral DG, Dolorfo C, Alvarez-Royo P. Organization of CA1 projections to the subiculum: a PHA-L analysis in the rat. Hippocampus. 1991;1:415–435. - PubMed
-
- Angrist B, Sathananthan G, Wilk S, Gershon S. Amphetamine psychosis: behavioral and biochemical aspects. Journal of Psychiatric Research. 1974;11:13–23. - PubMed
-
- Angrist B, Thompson H, Shopsin B, Gershon S. Clinical studies with dopamine-receptor stimulants. Psychopharmacologia. 1975;44:273–280. - PubMed
-
- Anstrom KK, Woodward DJ. Restraint increases dopaminergic burst firing in awake rats. Neuropsychopharmacology. 2005;30:1832–1840. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

