Quantification of hemodynamics in abdominal aortic aneurysms during rest and exercise using magnetic resonance imaging and computational fluid dynamics
- PMID: 20143263
- PMCID: PMC6203348
- DOI: 10.1007/s10439-010-9949-x
Quantification of hemodynamics in abdominal aortic aneurysms during rest and exercise using magnetic resonance imaging and computational fluid dynamics
Abstract
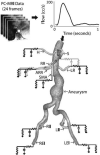
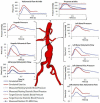
Abdominal aortic aneurysms (AAAs) affect 5-7% of older Americans. We hypothesize that exercise may slow AAA growth by decreasing inflammatory burden, peripheral resistance, and adverse hemodynamic conditions such as low, oscillatory shear stress. In this study, we use magnetic resonance imaging and computational fluid dynamics to describe hemodynamics in eight AAAs during rest and exercise using patient-specific geometric models, flow waveforms, and pressures as well as appropriately resolved finite-element meshes. We report mean wall shear stress (MWSS) and oscillatory shear index (OSI) at four aortic locations (supraceliac, infrarenal, mid-aneurysm, and suprabifurcation) and turbulent kinetic energy over the entire computational domain on meshes containing more than an order of magnitude more elements than previously reported results (mean: 9.0-million elements; SD: 2.3 M; range: 5.7-12.0 M). MWSS was lowest in the aneurysm during rest 2.5 dyn/cm(2) (SD: 2.1; range: 0.9-6.5), and MWSS increased and OSI decreased at all four locations during exercise. Mild turbulence existed at rest, while moderate aneurysmal turbulence was present during exercise. During both rest and exercise, aortic turbulence was virtually zero superior to the AAA for seven out of eight patients. We postulate that the increased MWSS, decreased OSI, and moderate turbulence present during exercise may attenuate AAA growth.
Figures







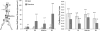


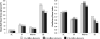

References
-
- Allardice JT, Allwright GJ, Wafula JM, Wyatt AP. High prevalence of abdominal aortic aneurysm in men with peripheral vascular disease: Screening by ultrasonography. Brit. J. Surg. 1988;75:240–2. - PubMed
-
- Asbury CL, Ruberti JW, Bluth EI, Peattie RA. Experimental investigation of steady flow in rigid models of abdominal aortic aneurysms. Ann Biomed Eng. 1995;23:29–39. - PubMed
-
- Bax L, Bakker CJ, Klein WM, Blanken N, Beutler JJ, Mali WP. Renal blood flow measurements with use of phase-contrast magnetic resonance imaging: Normal values and reproducibility. J. Vasc. Interv. Radiol. 2005;16:807–14. - PubMed
-
- Berguer R, Bull JL, Khanafer K. Refinements in mathematical models to predict aneurysm growth and rupture. Ann. N.Y. Acad. Sci. 2006;1085:110–6. - PubMed
-
- Bluestein D, Niu L, Schoephoerster RT, Dewanjee MK. Steady flow in an aneurysm model: Correlation between fluid dynamics and blood platelet deposition. Journal of Biomechamical Engineering. 1996;118:280–6. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

