Transgenic mouse and cell culture models demonstrate a lack of mechanistic connection between endoplasmic reticulum stress and tau dysfunction
- PMID: 20143409
- PMCID: PMC4560366
- DOI: 10.1002/jnr.22359
Transgenic mouse and cell culture models demonstrate a lack of mechanistic connection between endoplasmic reticulum stress and tau dysfunction
Abstract
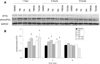
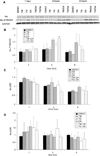
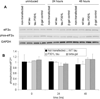
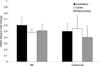
In vivo aggregation of tau protein is a hallmark of many neurodegenerative disorders, including Alzheimer's disease (AD). Recent evidence has also demonstrated activation of the unfolded protein response (UPR), a cellular response to endoplasmic reticulum (ER) stress, in AD, although the role of the UPR in disease pathogenesis is not known. Here, three model systems were used to determine whether a direct mechanistic link could be demonstrated between tau aggregation and the UPR. The first model system used was SH-SY5Y cells, a neuronal cultured cell line that endogenously expresses tau. In this system, the UPR was activated using chemical stressors, tunicamycin and thapsigargin, but no changes in tau expression levels, solubility, or phosphorylation were observed. In the second model system, wild-type 4R tau and P301L tau, a variant with increased aggregation propensity, were heterologously overexpressed in HEK 293 cells. This overexpression did not activate the UPR. The last model system examined here was the PS19 transgenic mouse model. Although PS19 mice, which express the P301S variant of tau, display severe neurodegeneration and formation of tau aggregates, brain tissue samples did not show any activation of the UPR. Taken together, the results from these three model systems suggest that a direct mechanistic link does not exist between tau aggregation and the UPR.
Figures





Similar articles
-
Pathogenic tau does not drive activation of the unfolded protein response.J Biol Chem. 2019 Jun 21;294(25):9679-9688. doi: 10.1074/jbc.RA119.008263. Epub 2019 May 3. J Biol Chem. 2019. PMID: 31053641 Free PMC article.
-
Bidirectional interplay of HSF1 degradation and UPR activation promotes tau hyperphosphorylation.PLoS Genet. 2017 Jul 5;13(7):e1006849. doi: 10.1371/journal.pgen.1006849. eCollection 2017 Jul. PLoS Genet. 2017. PMID: 28678786 Free PMC article.
-
Tau accumulation activates the unfolded protein response by impairing endoplasmic reticulum-associated degradation.J Neurosci. 2013 May 29;33(22):9498-507. doi: 10.1523/JNEUROSCI.5397-12.2013. J Neurosci. 2013. PMID: 23719816 Free PMC article.
-
Stress signaling from the endoplasmic reticulum: A central player in the pathogenesis of amyotrophic lateral sclerosis.IUBMB Life. 2011 Sep;63(9):754-63. doi: 10.1002/iub.520. Epub 2011 Aug 10. IUBMB Life. 2011. PMID: 21834058 Review.
-
Endoplasmic reticulum: the unfolded protein response is tangled in neurodegeneration.Int J Biochem Cell Biol. 2012 Aug;44(8):1295-8. doi: 10.1016/j.biocel.2012.04.023. Epub 2012 May 4. Int J Biochem Cell Biol. 2012. PMID: 22564438 Review.
Cited by
-
The PERK-Dependent Molecular Mechanisms as a Novel Therapeutic Target for Neurodegenerative Diseases.Int J Mol Sci. 2020 Mar 19;21(6):2108. doi: 10.3390/ijms21062108. Int J Mol Sci. 2020. PMID: 32204380 Free PMC article. Review.
-
ER-stress in Alzheimer's disease: turning the scale?Am J Neurodegener Dis. 2013 Nov 29;2(4):247-65. Am J Neurodegener Dis. 2013. PMID: 24319643 Free PMC article. Review.
-
Gene regulation and genetics in neurochemistry, past to future.J Neurochem. 2016 Oct;139 Suppl 2(Suppl 2):24-57. doi: 10.1111/jnc.13629. Epub 2016 Oct 17. J Neurochem. 2016. PMID: 27747882 Free PMC article. Review.
-
Pathogenic tau does not drive activation of the unfolded protein response.J Biol Chem. 2019 Jun 21;294(25):9679-9688. doi: 10.1074/jbc.RA119.008263. Epub 2019 May 3. J Biol Chem. 2019. PMID: 31053641 Free PMC article.
-
Critical review: involvement of endoplasmic reticulum stress in the aetiology of Alzheimer's disease.Open Biol. 2018 Apr;8(4):180024. doi: 10.1098/rsob.180024. Open Biol. 2018. PMID: 29695619 Free PMC article. Review.
References
-
- Alzheimer’s Association. 2009 Alzheimer’s disease facts and figures. Alzheimers Dementia. 2009;5 - PubMed
-
- Atkin JD, Farg MA, Walker AK, McLean C, Tomas D, Horne MK. Endoplasmic reticulum stress and induction of the unfolded protein response in human sporadic amyotrophic lateral sclerosis. Neuro-biol Dis. 2008;30:400–407. - PubMed
-
- Biedler JL, Roffler-Tarlov S, Schachner M, Freedman LS. Multiple neurotransmitter synthesis by human neuroblastoma cell lines and clones. Cancer Res. 1978;38:3751–3757. - PubMed
-
- Brandt R, Hundelt M, Shahani N. Tau alteration and neuronal degeneration in tauopathies: mechanisms and models. Biochim Biophys Acta. 2005;1739:331–354. - PubMed
-
- Brewster JL, Linseman DA, Bouchard RJ, Loucks FA, Precht TA, Esch EA, Heidenreich KA. Endoplasmic reticulum stress and trophic factor withdrawal activate distinct signaling cascades that induce glyco-gen synthase kinase-3 beta and a caspase-9-dependent apoptosis in cere-bellar granule neurons. Mol Cell Neurosci. 2006;32:242–253. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

