Single-institution experience with ipilimumab in advanced melanoma patients in the compassionate use setting: lymphocyte count after 2 doses correlates with survival
- PMID: 20143434
- PMCID: PMC2917065
- DOI: 10.1002/cncr.24951
Single-institution experience with ipilimumab in advanced melanoma patients in the compassionate use setting: lymphocyte count after 2 doses correlates with survival
Abstract
Background: : Ipilimumab is a monoclonal antibody that antagonizes cytotoxic T lymphocyte antigen-4, a negative regulator of the immune system. The authors report on advanced refractory melanoma patients treated in a compassionate use trial of ipilimumab at the Memorial Sloan-Kettering Cancer Center.
Methods: : Patients with advanced refractory melanoma were treated in a compassionate use trial with ipilimumab 10 mg/kg every 3 weeks for 4 doses. Those with evidence of clinical benefit at Week 24 (complete response [CR], partial response [PR], or stable disease [SD]) then received ipilimumab every 12 weeks.
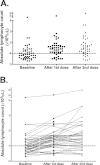
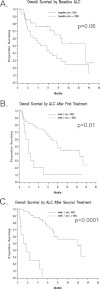
Results: : A total of 53 patients were enrolled, with 51 evaluable. Grade 3/4 immune-related adverse events were noted in 29% of patients, with the most common immune-related adverse events being pruritus (43%), rash (37%), and diarrhea (33%). On the basis of immune-related response criteria, the response rate (CR + PR) was 12% (95% confidence interval [CI], 5%-25%), whereas 29% had SD (95% CI, 18%-44%). The median progression-free survival was 2.6 months (95% CI, 2.3-5.2 months), whereas the median overall survival (OS) was 7.2 months (95% CI, 4.0-13.3 months). Patients with an absolute lymphocyte count (ALC) > micro =1000/microL after 2 ipilimumab treatments (Week 7) had a significantly improved clinical benefit rate (51% vs 0%; P = .01) and median OS (11.9 vs 1.4 months; P < .001) compared with those with an ALC <1000/microL.
Conclusions: : The results confirm that ipilimumab is clinically active in patients with advanced refractory melanoma. The ALC after 2 ipilimumab treatments appears to correlate with clinical benefit and OS, and should be prospectively validated. Cancer 2010. (c) 2010 American Cancer Society.
Figures
References
-
- American Cancer Society Cancer Facts and Figures, 2008. Available at: http://www.cancer.org/downloads/STT/2008CAFFfinalsecured.pdf Accessed June 30, 2009.
-
- Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin-2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105–2116. - PubMed
-
- Eigentler TK, Caroli UM, Radny P, Garbe C. Palliative therapy of disseminated malignant melanoma: a systematic review of 41 randomised clinical trials. Lancet Oncol. 2003;4:748–759. - PubMed
-
- Chapman PB, Einhorn LH, Meyers ML, et al. Phase III multicenter randomized trial of the Dartmouth regimen versus dacarbazine in patients with metastatic melanoma. J Clin Oncol. 1999;17:2745–2751. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

