Phase 2 trial of talampanel, a glutamate receptor inhibitor, for adults with recurrent malignant gliomas
- PMID: 20143438
- PMCID: PMC2846997
- DOI: 10.1002/cncr.24957
Phase 2 trial of talampanel, a glutamate receptor inhibitor, for adults with recurrent malignant gliomas
Abstract
Background: : Glioma cells secrete glutamate and also express alpha-amino-3-hydroxy-5 methyl-4-isoxazolepropionate (AMPA) glutamate receptors, which contribute to the proliferation, migration, and neurotoxicity of malignant gliomas. Talampanel is an oral AMPA receptor inhibitor with excellent central nervous system penetration and good tolerability in clinical trials for epilepsy and other neurologic disorders.
Methods: : A phase 2 trial was conducted to evaluate the efficacy of talampanel in patients with recurrent malignant glioma as measured by 6-month progression-free survival (PFS6).
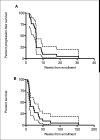
Results: : Thirty patients (22 with glioblastomas [GBMs] and 8 with anaplastic gliomas [AGs]; 63% men) with median age of 51 years (range, 20-67 years) and a median Karnofsky performance scale of 80 were included. Patients tolerated treatment well, and most adverse events were mild and reversible; the most common toxicities were fatigue (27%), dizziness (23%), and ataxia (17%). There was only 1 partial response (5%) reported in the GBM stratum and none among AG patients. At a median follow-up of 13 months, 28 patients (93%) had died. The PFS6 was 4.6% for the initial 22 GBM patients, and the study was terminated early due to treatment futility; the PFS6 was 0% for 8 AG patients. The median PFS was 5.9 weeks for GBM and 8.9 weeks for AG patients. The median overall survival was 13 weeks for GBM patients and 14 months for AG patients.
Conclusions: : Talampanel was well-tolerated but had no significant activity as a single agent in unselected recurrent malignant gliomas. Cancer 2010. Published 2010 by the American Cancer Society.
Figures
References
-
- CBTRUS 2008 statistical report: Primary Brain Tumors in the United States, 2000-2004 (years of data collected) Central Brain Tumor Registry of the United States. Available at http://www.cbtrus.org. Accessed on January 20, 2009.
-
- Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. - PubMed
-
- Takano T, Lin JH, Arcuino G, Gao Q, Yang J, Nedergaard M. Glutamate release promotes growth of malignant gliomas. Nat Med. 2001;7:1010–1015. - PubMed
-
- Ye ZC, Sontheimer H. Glioma cells release excitotoxic concentrations of glutamate. Cancer Res. 1999;59:4383–4391. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

