Silicon photonic microring resonators for quantitative cytokine detection and T-cell secretion analysis
- PMID: 20143780
- PMCID: PMC2843522
- DOI: 10.1021/ac902725q
Silicon photonic microring resonators for quantitative cytokine detection and T-cell secretion analysis
Abstract
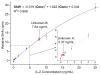
The ability to perform multiple simultaneous protein biomarker measurements in complex media with picomolar sensitivity presents a large challenge to disease diagnostics and fundamental biological studies. Silicon photonic microring resonators represent a promising platform for real-time detection of biomolecules on account of their spectral sensitivity toward surface binding events between a target and antibody-modified microrings. For all refractive index-based sensing schemes, the mass of bound analytes, in combination with other factors such as antibody affinity and surface density, contributes to the observed signal and measurement sensitivity. Therefore, proteins that are simultaneously low in abundance and have a lower molecular weight are often challenging to detect. By employing a more massive secondary antibody to amplify the signal arising from the initial binding event, it is possible to improve both the sensitivity and the specificity of protein assays, allowing for quantitative sensing in complex sample matrices. Herein, a sandwich assay is used to detect the 15.5 kDa human cytokine interleukin-2 (IL-2) at concentrations down to 100 pg/mL (6.5 pM) and to quantitate unknown solution concentrations over a dynamic range spanning 2.5 orders of magnitude. This same sandwich assay is then used to monitor the temporal secretion profile of IL-2 from Jurkat T lymphocytes in serum-containing cell culture media in the presence of the entire Jurkat secretome. The same temporal secretion analysis is performed in parallel using a commercial ELISA, revealing similar IL-2 concentration profiles but superior precision for the microring resonator sensing platform. Furthermore, we demonstrate the generality of the sandwich assay methodology on the microring resonator platform for the analysis of any biomolecular target for which two high-affinity antibodies exist by detecting the approximately 8 kDa cytokine interleukin-8 (IL-8) with a limit of detection and dynamic range similar to that of IL-2. This work demonstrates the first application of silicon photonic microring resonators for detecting cellular secretion of cytokines and represents an important advance for the detection of protein biomarkers on an emerging analytical platform.
Figures



Similar articles
-
Rapid, multiparameter profiling of cellular secretion using silicon photonic microring resonator arrays.J Am Chem Soc. 2011 Dec 21;133(50):20500-6. doi: 10.1021/ja2087618. Epub 2011 Nov 22. J Am Chem Soc. 2011. PMID: 22040005 Free PMC article.
-
Subpicogram per milliliter detection of interleukins using silicon photonic microring resonators and an enzymatic signal enhancement strategy.Anal Chem. 2013 Nov 19;85(22):10653-7. doi: 10.1021/ac402972d. Epub 2013 Nov 4. Anal Chem. 2013. PMID: 24171505
-
Sensitive on-chip detection of a protein biomarker in human serum and plasma over an extended dynamic range using silicon photonic microring resonators and sub-micron beads.Lab Chip. 2011 Jun 21;11(12):2042-4. doi: 10.1039/c1lc20231f. Epub 2011 May 4. Lab Chip. 2011. PMID: 21541438 Free PMC article.
-
[Research progress and clinical application potential of silicon-based microring resonators in biomedical detection].Sheng Wu Gong Cheng Xue Bao. 2025 Apr 25;41(4):1309-1322. doi: 10.13345/j.cjb.240702. Sheng Wu Gong Cheng Xue Bao. 2025. PMID: 40328698 Review. Chinese.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
Cited by
-
Biomolecular analysis with microring resonators: applications in multiplexed diagnostics and interaction screening.Curr Opin Chem Biol. 2013 Oct;17(5):818-26. doi: 10.1016/j.cbpa.2013.06.014. Epub 2013 Jul 17. Curr Opin Chem Biol. 2013. PMID: 23871688 Free PMC article. Review.
-
Biofunctionalization of Multiplexed Silicon Photonic Biosensors.Biosensors (Basel). 2022 Dec 29;13(1):53. doi: 10.3390/bios13010053. Biosensors (Basel). 2022. PMID: 36671887 Free PMC article. Review.
-
Interferometric methods for label-free molecular interaction studies.Anal Chem. 2012 Jan 17;84(2):779-92. doi: 10.1021/ac202812h. Epub 2011 Nov 7. Anal Chem. 2012. PMID: 22060037 Free PMC article. Review. No abstract available.
-
Multiparametric Biomechanical and Biochemical Phenotypic Profiling of Single Cancer Cells Using an Elasticity Microcytometer.Small. 2016 May;12(17):2300-11. doi: 10.1002/smll.201503620. Epub 2016 Mar 1. Small. 2016. PMID: 26929029 Free PMC article.
-
An integrated adipose-tissue-on-chip nanoplasmonic biosensing platform for investigating obesity-associated inflammation.Lab Chip. 2018 Dec 7;18(23):3550-3560. doi: 10.1039/c8lc00605a. Epub 2018 Oct 10. Lab Chip. 2018. PMID: 30302487 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

