Non-peptide small molecule regulators of lymphangiogenesis
- PMID: 20143917
- PMCID: PMC2843547
- DOI: 10.1089/lrb.2009.0033
Non-peptide small molecule regulators of lymphangiogenesis
Abstract
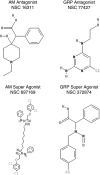
Adrenomedullin (AM) and gastrin releasing peptide (GRP) are neuroendocrine peptides that have been previously implicated as regulators of angiogenesis and lymphangiogenesis. Using an immortalized human dermal microvascular lymphatic endothelial cell line stably transfected with red fluorescent protein (LEC/RFP), we demonstrate the ability of AM and GRP to augment tube formation complexity of this target cell in a dose-dependent manner. Maximum tube density was initiated at 1 nM for both peptides, and as concentrations exceeded 10 nM a decrease in tube formation was noted, hence following a classic rise/fall biological response curve. In addition, we show that appropriate small molecule mimetics to neutralizing monoclonal antibodies of AM or GRP, at 1 microM concentration, can function to either inhibit (antagonist) or enhance (super agonist) peptide-induced tube formation of LEC/RFP. Our small molecule reagents by themselves have no activity, but in the presence of their respective peptides can mediate a positive or negative response, hence the super agonist designation. These compounds represent new regulatory drugs of the lymphatic system with possible patient application in the clinical management of edema and metastatic disease.
Figures




References
-
- Olszewski W. The lymphatic system in body homeostasis: Physiological conditions. Lymphat Res Biol. 2003;1:11–21. - PubMed
-
- Rocha SF. Adams RH. Molecular differentiation and specialization of vascular beds. Angiogenesis. 2009;12:139–147. - PubMed
-
- Wigle JT. Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999;98:769–778. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
