Lymphatic endothelial cells adapt their barrier function in response to changes in shear stress
- PMID: 20143922
- PMCID: PMC2883493
- DOI: 10.1089/lrb.2009.0015
Lymphatic endothelial cells adapt their barrier function in response to changes in shear stress
Abstract
Background: Lymphatic endothelial cells form an important barrier necessary for normal lymph formation and propulsion. However, little is known about how physical forces within lymphatic vessels affect endothelial barrier function. The purpose of this study was to characterize how laminar flow affects lymphatic endothelial barrier function and to test whether endothelial cells respond to flow changes by activating the intracellular actin cytoskeleton to enhance barrier function.
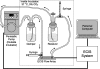
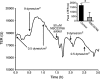
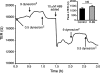
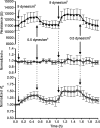
Methods and results: Cultured adult human dermal microlymphatic endothelial cells (HMLEC-d) were grown on small gold electrodes arranged within a flow channel, and transendothelial electrical resistance (TER), an index of barrier function, was determined. Laminar flow was applied to the cells at a baseline shear stress of 0.5 dynes/cm(2), and was increased to 2.5, 5.0, or 9.0 dynes/cm(2), causing a magnitude-dependent increase in barrier function that was reversed 30 min later when the shear stress was returned to baseline. This response was abolished by blockade of actin dynamics with 10 microM phalloidin, and significantly inhibited by blockade of Rac1 activity with 50 microM NSC23766. Blockade of protein kinase A (10 microM H-89) did not inhibit the response. Mathematical modeling based on our impedance data showed that the flow-induced changes in TER were primarily due to altered current flow between cells and not beneath cells.
Conclusions: These results suggest that lymphatic endothelial cells dynamically alter their morphology and barrier function in response to changes in shear stress by a mechanism dependent upon Rac1-mediated actin dynamics.
Figures
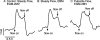



References
-
- Murfee WL. Rappleye JW. Ceballos M. Schmid–Schonbein GW. Discontinuous expression of endothelial cell adhesion molecules along initial lymphatic vessels in mesentery: the primary valve structure. Lymphat Res Biol. 2007;5:81–89. - PubMed
-
- Kuo L. Davis MJ. Chilian WM. Endothelium-dependent, flow-induced dilation of isolated coronary arterioles. Am J Physiol. 1990;259:H1063–H1070. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

