Review of meningococcal group B vaccines
- PMID: 20144017
- PMCID: PMC2820413
- DOI: 10.1086/648966
Review of meningococcal group B vaccines
Abstract
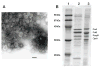
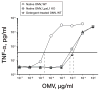
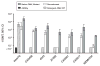
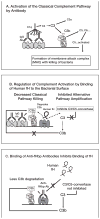
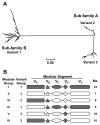
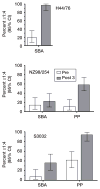
No broadly effective vaccines are available for prevention of group B meningococcal disease, which accounts for >50% of all cases. The group B capsule is an autoantigen and is not a suitable vaccine target. Outer-membrane vesicle vaccines appear to be safe and effective, but serum bactericidal responses in infants are specific for a porin protein, PorA, which is antigenically variable. To broaden protection, outer-membrane vesicle vaccines have been prepared from >1 strain, from mutants with >1 PorA, or from mutants with genetically detoxified endotoxin and overexpressed desirable antigens, such as factor H binding protein. Also, recombinant protein vaccines such as factor H binding protein, given alone or in combination with other antigens, are in late-stage clinical development and may be effective against the majority of group B strains. Thus, the prospects have never been better for developing vaccines for prevention of meningococcal disease, including that caused by group B strains.
Conflict of interest statement
Figures
References
-
- Harrison LH, Trotter CL, Ramsay ME. Global epidemiology of meningococcal disease. Vaccine. 2009;27:B51–B63. - PubMed
-
- Trotter CL, Chandra M, Cano R, et al. A surveillance network for meningococcal disease in Europe. FEMS Microbiol Rev. 2007;31:27–36. - PubMed
-
- Gray SJ, Trotter CL, Ramsay ME, et al. Epidemiology of meningococcal disease in England and Wales 1993/94 to 2003/04: contribution and experiences of the Meningococcal Reference Unit. J Med Microbiol. 2006;55:887–96. - PubMed
-
- Trotter CL, Andrews NJ, Kaczmarski EB, Miller E, Ramsay ME. Effectiveness of meningococcal serogroup C conjugate vaccine 4 years after introduction. Lancet. 2004;364:365–7. - PubMed
-
- Shepard CW, Rosenstein NE, Fischer M. Neonatal meningococcal disease in the United States, 1990 to 1999. Pediatr Infect Dis J. 2003;22:418–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

