Facilitation of synaptic transmission and pain responses by CGRP in the amygdala of normal rats
- PMID: 20144185
- PMCID: PMC2829526
- DOI: 10.1186/1744-8069-6-10
Facilitation of synaptic transmission and pain responses by CGRP in the amygdala of normal rats
Abstract
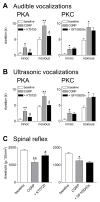
Calcitonin gene-related peptide (CGRP) plays an important role in peripheral and central sensitization. CGRP also is a key molecule in the spino-parabrachial-amygdaloid pain pathway. Blockade of CGRP1 receptors in the spinal cord or in the amygdala has antinociceptive effects in different pain models. Here we studied the electrophysiological mechanisms of behavioral effects of CGRP in the amygdala in normal animals without tissue injury.Whole-cell patch-clamp recordings of neurons in the latero-capsular division of the central nucleus of the amygdala (CeLC) in rat brain slices showed that CGRP (100 nM) increased excitatory postsynaptic currents (EPSCs) at the parabrachio-amygdaloid (PB-CeLC) synapse, the exclusive source of CGRP in the amygdala. Consistent with a postsynaptic mechanism of action, CGRP increased amplitude, but not frequency, of miniature EPSCs and did not affect paired-pulse facilitation. CGRP also increased neuronal excitability. CGRP-induced synaptic facilitation was reversed by an NMDA receptor antagonist (AP5, 50 microM) or a PKA inhibitor (KT5720, 1 microM), but not by a PKC inhibitor (GF109203X, 1 microM). Stereotaxic administration of CGRP (10 microM, concentration in microdialysis probe) into the CeLC by microdialysis in awake rats increased audible and ultrasonic vocalizations and decreased hindlimb withdrawal thresholds. Behavioral effects of CGRP were largely blocked by KT5720 (100 microM) but not by GF109203X (100 microM).The results show that CGRP in the amygdala exacerbates nocifensive and affective behavioral responses in normal animals through PKA- and NMDA receptor-dependent postsynaptic facilitation. Thus, increased CGRP levels in the amygdala might trigger pain in the absence of tissue injury.
Figures





Similar articles
-
Critical role of calcitonin gene-related peptide 1 receptors in the amygdala in synaptic plasticity and pain behavior.J Neurosci. 2005 Nov 16;25(46):10717-28. doi: 10.1523/JNEUROSCI.4112-05.2005. J Neurosci. 2005. PMID: 16291945 Free PMC article.
-
Non-pain-related CRF1 activation in the amygdala facilitates synaptic transmission and pain responses.Mol Pain. 2013 Feb 15;9:2. doi: 10.1186/1744-8069-9-2. Mol Pain. 2013. PMID: 23410057 Free PMC article.
-
PKA and ERK, but not PKC, in the amygdala contribute to pain-related synaptic plasticity and behavior.Mol Pain. 2008 Jul 16;4:26. doi: 10.1186/1744-8069-4-26. Mol Pain. 2008. PMID: 18631385 Free PMC article.
-
Enhanced group II mGluR-mediated inhibition of pain-related synaptic plasticity in the amygdala.Mol Pain. 2006 May 8;2:18. doi: 10.1186/1744-8069-2-18. Mol Pain. 2006. PMID: 16681859 Free PMC article.
-
Pain-related synaptic plasticity in spinal dorsal horn neurons: role of CGRP.Mol Pain. 2006 Sep 26;2:31. doi: 10.1186/1744-8069-2-31. Mol Pain. 2006. PMID: 17002803 Free PMC article.
Cited by
-
Anxiogenic effects of CGRP within the BNST may be mediated by CRF acting at BNST CRFR1 receptors.Behav Brain Res. 2013 Apr 15;243:286-93. doi: 10.1016/j.bbr.2013.01.024. Epub 2013 Feb 1. Behav Brain Res. 2013. PMID: 23376701 Free PMC article.
-
α(2A)-adrenergic receptors filter parabrachial inputs to the bed nucleus of the stria terminalis.J Neurosci. 2014 Jul 9;34(28):9319-31. doi: 10.1523/JNEUROSCI.0822-14.2014. J Neurosci. 2014. PMID: 25009265 Free PMC article.
-
TRP Channel Agonists Activate Different Afferent Neuromodulatory Mechanisms in Guinea Pig Urinary Bladder.Front Physiol. 2021 Jun 24;12:692719. doi: 10.3389/fphys.2021.692719. eCollection 2021. Front Physiol. 2021. PMID: 34248678 Free PMC article.
-
Pain-related increase of excitatory transmission and decrease of inhibitory transmission in the central nucleus of the amygdala are mediated by mGluR1.Mol Pain. 2010 Dec 16;6:93. doi: 10.1186/1744-8069-6-93. Mol Pain. 2010. PMID: 21162731 Free PMC article.
-
Elucidating an Affective Pain Circuit that Creates a Threat Memory.Cell. 2015 Jul 16;162(2):363-374. doi: 10.1016/j.cell.2015.05.057. Cell. 2015. PMID: 26186190 Free PMC article.
References
-
- Wimalawansa SJ. Calcitonin gene-related peptide and its receptors: molecular genetics, physiology, pathophysiology, and therapeutic potentials. Endocr Rev. 1996;17:533–585. - PubMed
-
- Poyner DR, Sexton PM, Marshall I, Smith DM, Quirion R, Born W, Muff R, Fischer JA, Foord SM. International Union of Pharmacology. XXXII. The mammalian calcitonin gene-related peptides, adrenomedullin, amylin, and calcitonin receptors. Pharmacol Rev. 2002;54:233–246. doi: 10.1124/pr.54.2.233. - DOI - PubMed
-
- Van Rossum D, Hanish U-K, Quirion R. Neuroanatomical Localization, Pharmacological Characterization and Functions of CGRP, Related Peptides and Their Receptors. Neuroscience & Biobehavioral Reviews. 1997;21:649–678. - PubMed
-
- Neugebauer V. In: Synaptic Plasticity in Pain. Malcangio M, editor. New York: Springer; 2009. CGRP in Spinal Cord Pain Mechanisms; pp. 175–197. full_text.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

