A candidate probiotic with unfavourable effects in subjects with irritable bowel syndrome: a randomised controlled trial
- PMID: 20144246
- PMCID: PMC2831047
- DOI: 10.1186/1471-230X-10-16
A candidate probiotic with unfavourable effects in subjects with irritable bowel syndrome: a randomised controlled trial
Abstract
Background: Some probiotics have shown efficacy for patients with irritable bowel syndrome (IBS). Lactobacillus (L.) plantarum MF1298 was found to have the best in vitro probiotic properties of 22 strains of lactobacilli. The aim of this study was to investigate the symptomatic effect of L. plantarum MF1298 in subjects with IBS. Primary outcome was treatment preference and secondary outcomes were number of weeks with satisfactory relief of symptoms and IBS sum score.
Methods: The design was a randomised double blind placebo-controlled crossover trial. 16 subjects with IBS underwent two three-week periods of daily intake of one capsule of 10(10) CFU L. plantarum MF 1298 or placebo separated by a four-week washout period.
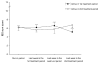
Results: Thirteen participants (81%; 95% CI 57% to 93%; P = 0.012) preferred placebo to L. plantarum MF1298 treatment. The mean (SD) number of weeks with satisfactory relief of symptoms in the periods with L. plantarum MF1298 and placebo were 0.50 (0.89) and 1.44 (1.26), respectively (P = 0.006). IBS sum score was 6.44 (1.81) in the period with L. plantarum MF1298 treatment compared with 5.35 (1.77) in the period with placebo (P = 0.010). With a clinically significant difference in the IBS sum score of 2 in disfavour of active treatment, the number needed to harm was 3.7, 95% CI 2.3 to 10.9.
Conclusions: This trial shows for the first time an unfavourable effect on symptoms in subjects with IBS after intake of a potential probiotic.
Trial registration: ClinicalTrials.gov NCT00355810.
Figures
References
-
- FAO/WHO Expert Consultation Group. Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Argentina: FAO/WHO; 2001.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical