Noninvasive ultrasonic glucose sensing with large pigs (approximately 200 pounds) using a lightweight cymbal transducer array and biosensors
- PMID: 20144290
- PMCID: PMC2769881
- DOI: 10.1177/193229680900300316
Noninvasive ultrasonic glucose sensing with large pigs (approximately 200 pounds) using a lightweight cymbal transducer array and biosensors
Abstract
Background: To prevent complications in diabetes, the proper management of blood glucose levels is essential. Since conventional glucose meters require pricking fingers or other areas of the skin, a noninvasive method for monitoring blood glucose levels is desired. Using a lightweight cymbal transducer array, this study was conducted to noninvasively determine the glucose levels of pigs having a similar size to humans.
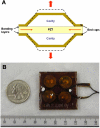
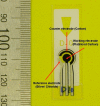
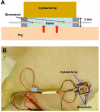
Method: In vivo experiments using eight pigs (approximately 200 pounds) were performed in five groups. A cymbal array with four biosensors was attached to the axillary area of the pig. The array was operated at 20 kHz at special peak-temporal peak intensity (I(sptp)) equal to 50 or 100 mW/cm(2) for 5, 10, or 20 minutes. After the ultrasound exposure, glucose concentrations of the interstitial fluid were determined using biosensors. For comparison, glucose levels of blood samples collected from the ear vein were measured by a commercial glucose meter.
Result: In comparison, glucose levels determined by a cymbal array and biosensor system were close to those measured by a glucose meter. After a 20-minute ultrasound exposure at I(sptp) = 100 mW/cm(2), the average glucose level determined by the ultrasound system was 175 +/- 7 mg/dl, which is close to 166 +/- 5 mg/dl measured by the glucose meter.
Conclusion: Results indicate the feasibility of using a cymbal array for noninvasive glucose sensing on pigs having a similar size to humans. Further studies on the ultrasound conditions, such as frequency, intensity, and exposure time, will be continued for effective glucose sensing.
2009 Diabetes Technology Society.
Figures
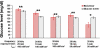
Similar articles
-
Glucose measurements with sensors and ultrasound.Ultrasound Med Biol. 2005 Jul;31(7):971-7. doi: 10.1016/j.ultrasmedbio.2005.04.004. Ultrasound Med Biol. 2005. PMID: 15972203
-
Ultrasound mediated transdermal insulin delivery in pigs using a lightweight transducer.Pharm Res. 2007 Jul;24(7):1396-401. doi: 10.1007/s11095-007-9306-4. Epub 2007 Apr 19. Pharm Res. 2007. PMID: 17443398
-
Noninvasive ultrasonic transdermal insulin delivery in rabbits using the light-weight cymbal array.Diabetes Technol Ther. 2004 Dec;6(6):808-15. doi: 10.1089/dia.2004.6.808. Diabetes Technol Ther. 2004. PMID: 15684633
-
A technology roadmap of smart biosensors from conventional glucose monitoring systems.Ther Deliv. 2017 Jun;8(6):411-423. doi: 10.4155/tde-2017-0012. Ther Deliv. 2017. PMID: 28530149 Review.
-
Advanced biosensors for glucose and insulin.Biosens Bioelectron. 2019 Sep 15;141:111201. doi: 10.1016/j.bios.2019.03.034. Epub 2019 Mar 19. Biosens Bioelectron. 2019. PMID: 31302426 Review.
Cited by
-
Applicability and safety of dual-frequency ultrasonic treatment for the transdermal delivery of drugs.J Control Release. 2015 Mar 28;202:93-100. doi: 10.1016/j.jconrel.2015.02.002. Epub 2015 Feb 4. J Control Release. 2015. PMID: 25662228 Free PMC article.
-
Skin permeabilization for transdermal drug delivery: recent advances and future prospects.Expert Opin Drug Deliv. 2014 Mar;11(3):393-407. doi: 10.1517/17425247.2014.875528. Epub 2014 Jan 7. Expert Opin Drug Deliv. 2014. PMID: 24392787 Free PMC article. Review.
-
Ultrasound-mediated transdermal drug delivery: mechanisms, scope, and emerging trends.J Control Release. 2011 Jun 30;152(3):330-48. doi: 10.1016/j.jconrel.2011.01.006. Epub 2011 Jan 14. J Control Release. 2011. PMID: 21238514 Free PMC article. Review.
-
Low-frequency sonophoresis: application to the transdermal delivery of macromolecules and hydrophilic drugs.Expert Opin Drug Deliv. 2010 Dec;7(12):1415-32. doi: 10.1517/17425247.2010.538679. Expert Opin Drug Deliv. 2010. PMID: 21118031 Free PMC article. Review.
-
Defining optimal permeant characteristics for ultrasound-mediated gastrointestinal delivery.J Control Release. 2017 Dec 28;268:113-119. doi: 10.1016/j.jconrel.2017.10.023. Epub 2017 Oct 16. J Control Release. 2017. PMID: 29051063 Free PMC article.
References
-
- The Diabetes Control and Complications Trial Research Group (DCCT) The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes-mellitus. N Engl J Med. 1993;329(14):977–986. - PubMed
-
- Wickramasinghe Y, Yang Y, Spencer SA. Current problems and potential techniques in in vivo glucose monitoring. J Fluoresc. 2004;14(5):513–520. - PubMed
-
- Sekkat N, Naik A, Kalia YN, Glikfeld P, Guy RH. Reverse iontophoretic monitoring in premature neonates: feasibility and potential. J Controlled Release. 2002;81(1-2):83–89. - PubMed
-
- Heinemann L. Continuous glucose monitoring by means of the microdialysis technique: underlying fundamental aspects. Diabetes Technol Ther. 2003;5(4):545–561. - PubMed
-
- Caduff A, Hirt E, Feldman Y, Ali Z, Heinemann L. First human experiments with a novel non-invasive, non-optical continuous glucose monitoring system. Biosens Bioelectron. 2003;19(3):209–217. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

