Usefulness of point-of-care testing in the treatment of diabetes in an underserved population
- PMID: 20144311
- PMCID: PMC2769936
- DOI: 10.1177/193229680900300409
Usefulness of point-of-care testing in the treatment of diabetes in an underserved population
Abstract
Background: The purpose of this article was to communicate our experience with point-of-care testing (POCT) using Bayer's A1CNow+ device to test glycated hemoglobin (A1C) in the management of diabetes and to share the observations of our quality control efforts.
Methods: Forty-seven patients' POCT samples were compared with laboratory samples to determine the validity of the POCT sample being drawn. Data collected represent a 10-month time period that were drawn on-site with the following distribution: 36 samples were drawn the same day, 7 samples were drawn 1 day later, 3 samples were drawn within 3 days, and 1 sample was drawn 4 days later. Although all samples were collected on-site, some of the samples were sent to other local branches of nationally recognized laboratories for analysis.
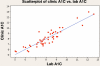
Results: The range of A1C results for the POCT group was 5.6 to >13%. The range of A1C results for the laboratory-drawn group was 5 to 12.6%. Twenty-four patients had laboratory results that read lower than the result obtained in the clinic, with an A1C range of 5 to 12.6%, and two patients had laboratory results that read exactly the same as the result obtained in the clinic when using POCT. These two individuals had A1C results of 9.1 and 12.6%. Analysis of data collected determined an r value of 0.918 demonstrating agreement between the POCT samples and the laboratory samples.
Conclusions: POCT with the A1CNow+ is an effective, economical tool for use in a pharmacist-based diabetes clinic that serves a high-risk underserved population. POCT allows the pharmacist the ability to use on-site results to inform patients of their progress, modify their therapy immediately with an immediate face-to-face opportunity to assure understanding, and provide a self-management goal.
Copyright 2009 Diabetes Technology Society.
Figures
Similar articles
-
Accuracy and clinical utility of a point-of-care HbA1c testing device.Postgrad Med. 2013 May;125(3):91-8. doi: 10.3810/pgm.2013.05.2664. Postgrad Med. 2013. PMID: 23748510
-
Implementing point-of-care hemoglobin A1C testing in an obstetrics outpatient clinic.Lab Med. 2024 Jul 3;55(4):464-470. doi: 10.1093/labmed/lmad112. Lab Med. 2024. PMID: 38217552
-
Impact of Point of Care Hemoglobin A1c Testing on Time to Therapeutic Intervention.J Am Board Fam Med. 2024 Jul-Aug;37(4):790-791. doi: 10.3122/jabfm.2023.230425R1. J Am Board Fam Med. 2024. PMID: 39455264
-
Point-of-Care Hemoglobin A1c Testing: An Evidence-Based Analysis.Ont Health Technol Assess Ser. 2014 Jul 1;14(8):1-30. eCollection 2014. Ont Health Technol Assess Ser. 2014. PMID: 26316922 Free PMC article. Review.
-
Point-of-Care Testing.J Pharm Pract. 2017 Apr;30(2):229-237. doi: 10.1177/0897190015587696. Epub 2016 Jul 9. J Pharm Pract. 2017. PMID: 26092752 Review.
Cited by
-
Community-based diagnosis of non-communicable diseases and their risk factors in rural and urban Haiti: a cross-sectional prevalence study.BMJ Open. 2018 Apr 20;8(4):e020317. doi: 10.1136/bmjopen-2017-020317. BMJ Open. 2018. PMID: 29678978 Free PMC article.
-
Utility of a point-of-care device in recruiting ethnic minorities for diabetes research with community partners.J Health Care Poor Underserved. 2011 Nov;22(4):1253-63. doi: 10.1353/hpu.2011.0117. J Health Care Poor Underserved. 2011. PMID: 22080707 Free PMC article.
-
Validity of a point of care device (Cholestech LDX) to monitor liver enzyme activity (aminotransferase measures) during a clinical trial.Contemp Clin Trials. 2010 Jul;31(4):279-82. doi: 10.1016/j.cct.2010.04.001. Epub 2010 Apr 27. Contemp Clin Trials. 2010. PMID: 20430116 Free PMC article.
-
Community-based type 2 diabetes screening programmes designed for priority populations: a scoping review protocol.BMJ Open. 2025 Jun 23;15(6):e090787. doi: 10.1136/bmjopen-2024-090787. BMJ Open. 2025. PMID: 40550708 Free PMC article.
-
HbA1c Performance in African Descent Populations in the United States With Normal Glucose Tolerance, Prediabetes, or Diabetes: A Scoping Review.Prev Chronic Dis. 2021 Mar 11;18:E22. doi: 10.5888/pcd18.200365. Prev Chronic Dis. 2021. PMID: 33705304 Free PMC article.
References
-
- American Diabetes Association (ADA) Diabetes statistics. Available from: http://www.diabetes.org/diabetes-statistics.jsp.
-
- USA Today. Study: cost of diabetes $218B. Available from: http://www.usatoday.com/news/health/2008-11-18-diabetes-cost_N.htm.
-
- Kost GJ. Guidelines for point-of-care testing. Improving patient outcomes. Am J Clin Pathol. 1995;104(4 Suppl 1):S111–S127. - PubMed
-
- Barr VJ, Robinson S, Marin-Link B, Underhill L, Dotts A, Ravensdale D, Salivaras S. The expanded Chronic Care Model: an integration of concepts and strategies from population health promotion and the Chronic Care Model. Hosp Quart. 2003;7(1):73–82. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical