Noninvasive glucose monitoring: a novel approach
- PMID: 20144356
- PMCID: PMC2771521
- DOI: 10.1177/193229680900300205
Noninvasive glucose monitoring: a novel approach
Abstract
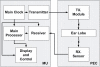
Background: The main concern in noninvasive (NI) glucose measurement is achieving high accuracy readings, although no blood (or other fluid) is involved in the process. Using methods based on different physical properties of a measured object can ensure the independence of each of the readings and therefore improve the validity of the end result. By using a combination of (three) independent technologies-ultrasonic, electromagnetic, and thermal-GlucoTrack presents a unique approach for a real-time, truly NI blood glucose spot measurement.
Methods: Clinical trials were performed in two stages. Stage 1 was an initial method validation and performance verification of the device. In this stage, 50 type 1 and 2 diabetic patients, as well as healthy subjects, were evaluated with GlucoTrack against Ascensia Elite (Bayer). In the second stage, 85 additional diabetic subjects were evaluated in half and full daytime sessions using a GlucoTrack comparison with HemoCue (Glucose 201+).
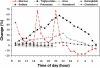
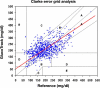
Results: A total of 135 subjects were tested during the trial period, producing 793 data pairs. Using Clarke error grid analysis, 92% of the readings fell in the clinically acceptable zones A and B, with 50% in the A zone. Mean and median relative absolute differences were 29.9 and 19.9%, respectively.
Conclusions: Integrating several modalities for NI assessment of glucose level enables more accurate readings, while a possible aberration in one modality is bypassed by the others. The present generation of GlucoTrack gives promising results; however, further improvement of the accuracy of the device is needed.
(c) 2009 Diabetes Technology Society.
Figures





References
-
- American Diabetes Association. Economic costs of diabetes in the U.S. in 2007. Diabetes Care. 2008;31(3):1–20. - PubMed
-
- DCCT Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. - PubMed
-
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in subjects with type 2 diabetes (UKPDS33) Lancet. 1998;352(9131):837–853. - PubMed
-
- Clinical Practice Recommendations. Standards of medical care in diabetes–2006 American Diabetes Association. Diabetes Care. 2006;29:S4–S42. - PubMed
-
- Florence JA, Yeager BF. Treatment of type 2 diabetes mellitus. Am Fam Physician. 1999;59(10):2835–2844. 2849-50. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

