Toward an agent-based patient-physician model for the adoption of continuous glucose monitoring technology
- PMID: 20144367
- PMCID: PMC2771527
- DOI: 10.1177/193229680900300217
Toward an agent-based patient-physician model for the adoption of continuous glucose monitoring technology
Abstract
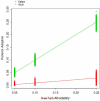
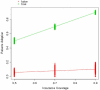
Health care is a major component of the U.S. economy, and tremendous research and development efforts are directed toward new technologies in this arena. Unfortunately few tools exist for predicting outcomes associated with new medical products, including whether new technologies will find widespread use within the target population. Questions of technology adoption are rife within the diabetes technology community, and we particularly consider the long-term prognosis for continuous glucose monitoring (CGM) technology. We present an approach to the design and analysis of an agent model that describes the process of CGM adoption among patients with type 1 diabetes mellitus (T1DM), their physicians, and related stakeholders. We particularly focus on patient-physician interactions, with patients discovering CGM technology through word-of-mouth communication and through advertising, applying pressure to their physicians in the context of CGM device adoption, and physicians, concerned about liability, looking to peers for a general level of acceptance of the technology before recommending CGM to their patients. Repeated simulation trials of the agent-based model show that the adoption process reflects the heterogeneity of the adopting community. We also find that the effect of the interaction between patients and physicians is agents. Each physician, say colored by the nature of the environment as defined by the model parameters. We find that, by being able to represent the diverse perspectives of different types of stakeholders, agent-based models can offer useful insights into the adoption process. Models of this sort may eventually prove to be useful in helping physicians, other health care providers, patient advocacy groups, third party payers, and device manufacturers understand the impact of their decisions about new technologies.
(c) 2009 Diabetes Technology Society.
Figures

Similar articles
-
Using Cluster Analysis to Understand Clinician Readiness to Promote Continuous Glucose Monitoring Adoption.J Diabetes Sci Technol. 2018 Nov;12(6):1108-1115. doi: 10.1177/1932296818786486. Epub 2018 Jul 11. J Diabetes Sci Technol. 2018. PMID: 29991281 Free PMC article.
-
Clinical application of emerging sensor technologies in diabetes management: consensus guidelines for continuous glucose monitoring (CGM).Diabetes Technol Ther. 2008 Aug;10(4):232-44; quiz 245-6. doi: 10.1089/dia.2008.0016. Diabetes Technol Ther. 2008. PMID: 18699743 Review.
-
Exploring Parental Experiences of Using a Do-It-Yourself Solution for Continuous Glucose Monitoring Among Children and Adolescents With Type 1 Diabetes: A Qualitative Study.J Diabetes Sci Technol. 2020 Sep;14(5):844-853. doi: 10.1177/1932296819895290. Epub 2019 Dec 25. J Diabetes Sci Technol. 2020. PMID: 31875411 Free PMC article.
-
Diabetes technology and treatments in the paediatric age group.Int J Clin Pract Suppl. 2011 Feb;(170):76-82. doi: 10.1111/j.1742-1241.2010.02582.x. Int J Clin Pract Suppl. 2011. PMID: 21323816 Review.
-
Nonadjunctive Use of Continuous Glucose Monitoring for Diabetes Treatment Decisions.J Diabetes Sci Technol. 2016 Aug 22;10(5):1169-73. doi: 10.1177/1932296816631569. Print 2016 Sep. J Diabetes Sci Technol. 2016. PMID: 26880390 Free PMC article. Review.
Cited by
-
Agent-Based Modeling in Public Health: Current Applications and Future Directions.Annu Rev Public Health. 2018 Apr 1;39:77-94. doi: 10.1146/annurev-publhealth-040617-014317. Epub 2018 Jan 12. Annu Rev Public Health. 2018. PMID: 29328870 Free PMC article. Review.
-
Informatics technology mimics ecology: dense, mutualistic collaboration networks are associated with higher publication rates.PLoS One. 2012;7(1):e30463. doi: 10.1371/journal.pone.0030463. Epub 2012 Jan 18. PLoS One. 2012. PMID: 22279593 Free PMC article.
-
Analyzing Medical Guideline Dissemination Behaviors Using Culturally Infused Agent Based Modeling Framework.IEEE J Biomed Health Inform. 2021 Jun;25(6):2137-2149. doi: 10.1109/JBHI.2021.3052809. Epub 2021 Jun 3. IEEE J Biomed Health Inform. 2021. PMID: 33465031 Free PMC article.
-
Agent-based modeling of noncommunicable diseases: a systematic review.Am J Public Health. 2015 Mar;105(3):e20-31. doi: 10.2105/AJPH.2014.302426. Epub 2015 Jan 20. Am J Public Health. 2015. PMID: 25602871 Free PMC article.
References
-
- Beasley D. Inhaled insulin flops, but field may hold promise. Reuters. http://www.reuters.com/article/healthNewsidUSN1344143820080413. 2008. Accessed November 28, 2008.
-
- Pierson R. Eli Lilly drops inhaled insulin program. Reuters. http://www.reuters.com/article/healthNews/idUSN0732561420080307. 2008. Accessed November 28, 2008.
-
- U.S. Food and Drug Administration. FDA approves first ever inhaled insulin combination product for treatment of diabetes. http://www.fda.gov/bbs/topics/news/2006/NEW01304.html. 2006. Accessed November 28, 2008.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

