Control to range for diabetes: functionality and modular architecture
- PMID: 20144419
- PMCID: PMC2769910
- DOI: 10.1177/193229680900300509
Control to range for diabetes: functionality and modular architecture
Abstract
Background: Closed-loop control of type 1 diabetes is receiving increasing attention due to advancement in glucose sensor and insulin pump technology. Here the function and structure of a class of control algorithms designed to exert control to range, defined as insulin treatment optimizing glycemia within a predefined target range by preventing extreme glucose fluctuations, are studied.
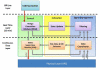
Methods: The main contribution of the article is definition of a modular architecture for control to range. Emphasis is on system specifications rather than algorithmic realization. The key system architecture elements are two interacting modules: range correction module, which assesses the risk for incipient hyper- or hypoglycemia and adjusts insulin rate accordingly, and safety supervision module, which assesses the risk for hypoglycemia and attenuates or discontinues insulin delivery when necessary. The novel engineering concept of range correction module is that algorithm action is relative to a nominal open-loop strategy-a predefined combination of basal rate and boluses believed to be optimal under nominal conditions.
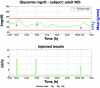
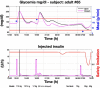
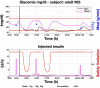
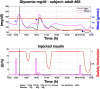
Results: A proof of concept of the feasibility of our control-to-range strategy is illustrated by using a prototypal implementation tested in silico on patient use cases. These functional and architectural distinctions provide several advantages, including (i) significant insulin delivery corrections are only made if relevant risks are detected; (ii) drawbacks of integral action are avoided, e.g., undershoots with consequent hypoglycemic risks; (iii) a simple linear model is sufficient and complex algorithmic constraints are replaced by safety supervision; and (iv) the nominal profile provides straightforward individualization for each patient.
Conclusions: We believe that the modular control-to-range system is the best approach to incremental development, regulatory approval, industrial deployment, and clinical acceptance of closed-loop control for diabetes.
2009 Diabetes Technology Society.
Figures
References
-
- Cryer PE. Hypoglycaemia: the limiting factor in the glycaemic management of Type I and type II diabetes. Diabetologia. 2002;45(7):937–948. - PubMed
-
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications of insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):978–986. - PubMed
-
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352(9131):837–853. - PubMed
-
- Clemens AH, Chang PH, Myers RW. The development of Biostator, a Glucose Controlled Insulin Infusion System (GCIIS) Horm Metab Res. 1977;(Suppl 7):23–33. - PubMed
-
- Steil GM, Rebrin K, Darwin C, Hariri F, Saad MF. Feasibility of automating insulin delivery for the treatment of type 1 diabetes. Diabetes. 2006;55(12):3344–3350. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

