Blood glucose controller for neonatal intensive care: virtual trials development and first clinical trials
- PMID: 20144420
- PMCID: PMC2769904
- DOI: 10.1177/193229680900300510
Blood glucose controller for neonatal intensive care: virtual trials development and first clinical trials
Abstract
Background: Premature neonates often experience hyperglycemia, which has been linked to worsened outcomes. Insulin therapy can assist in controlling blood glucose (BG) levels. However, a reliable, robust control protocol is required to avoid hypoglycemia and to ensure that clinically important nutrition goals are met.
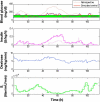
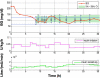
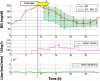
Methods: This study presents an adaptive, model-based predictive controller designed to incorporate the unique metabolic state of the neonate. Controller performance was tested and refined in virtual trials on a 25-patient retrospective cohort. The effects of measurement frequency and BG sensor error were evaluated. A stochastic model of insulin sensitivity was used in control to provide a guaranteed maximum 4% risk of BG < 72 mg/dl to protect against hypoglycemia as well as account for patient variability over 1-3 h intervals when determining the intervention. The resulting controller is demonstrated in two 24 h clinical neonatal pilot trials at Christchurch Women's Hospital.
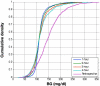
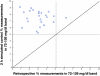
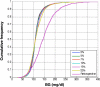
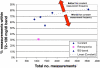
Results: Time in the 72-126 mg/dl BG band was increased by 103-161% compared to retrospective clinical control for virtual trials of the controller, with fewer hypoglycemic measurements. Controllers were robust to BG sensor errors. The model-based controller maintained glycemia to a tight target control range and accounted for interpatient variability in patient glycemic response despite using more insulin than the retrospective case, illustrating a further measure of controller robustness. Pilot clinical trials demonstrated initial safety and efficacy of the control method.
Conclusions: A controller was developed that made optimum use of the very limited available BG measurements in the neonatal intensive care unit and provided robustness against BG sensor error and longer BG measurement intervals. It used more insulin than typical sliding scale approaches or retrospective hospital control. The potential advantages of a model-based approach demonstrated in simulation were applied to initial clinical trials.
2009 Diabetes Technology Society.
Figures



Similar articles
-
On the problem of patient-specific endogenous glucose production in neonates on stochastic targeted glycemic control.J Diabetes Sci Technol. 2013 Jul 1;7(4):913-27. doi: 10.1177/193229681300700414. J Diabetes Sci Technol. 2013. PMID: 23911173 Free PMC article. Review.
-
Development of blood glucose control for extremely premature infants.Comput Methods Programs Biomed. 2011 May;102(2):181-91. doi: 10.1016/j.cmpb.2010.03.010. Epub 2011 Jan 17. Comput Methods Programs Biomed. 2011. PMID: 21247652
-
Comparative Simulation Study of Glucose Control Methods Designed for Use in the Intensive Care Unit Setting via a Novel Controller Scoring Metric.J Diabetes Sci Technol. 2017 Nov;11(6):1207-1217. doi: 10.1177/1932296817711297. Epub 2017 Jun 22. J Diabetes Sci Technol. 2017. PMID: 28637358 Free PMC article.
-
Stochastic targeted (STAR) glycemic control: design, safety, and performance.J Diabetes Sci Technol. 2012 Jan 1;6(1):102-15. doi: 10.1177/193229681200600113. J Diabetes Sci Technol. 2012. PMID: 22401328 Free PMC article.
-
Clinical need for continuous glucose monitoring in the hospital.J Diabetes Sci Technol. 2009 Nov 1;3(6):1309-18. doi: 10.1177/193229680900300611. J Diabetes Sci Technol. 2009. PMID: 20144385 Free PMC article. Review.
Cited by
-
On the problem of patient-specific endogenous glucose production in neonates on stochastic targeted glycemic control.J Diabetes Sci Technol. 2013 Jul 1;7(4):913-27. doi: 10.1177/193229681300700414. J Diabetes Sci Technol. 2013. PMID: 23911173 Free PMC article. Review.
-
Next-generation, personalised, model-based critical care medicine: a state-of-the art review of in silico virtual patient models, methods, and cohorts, and how to validation them.Biomed Eng Online. 2018 Feb 20;17(1):24. doi: 10.1186/s12938-018-0455-y. Biomed Eng Online. 2018. PMID: 29463246 Free PMC article. Review.
-
The Virtual Anemia Trial: An Assessment of Model-Based In Silico Clinical Trials of Anemia Treatment Algorithms in Patients With Hemodialysis.CPT Pharmacometrics Syst Pharmacol. 2018 Apr;7(4):219-227. doi: 10.1002/psp4.12276. Epub 2018 Jan 31. CPT Pharmacometrics Syst Pharmacol. 2018. PMID: 29368434 Free PMC article.
-
Continuous Glucose Monitoring in Transient Neonatal Diabetes Mellitus-2 Case Reports and Literature Review.Diagnostics (Basel). 2023 Jul 4;13(13):2271. doi: 10.3390/diagnostics13132271. Diagnostics (Basel). 2023. PMID: 37443665 Free PMC article. Review.
-
Pilot study of a model-based approach to blood glucose control in very-low-birthweight neonates.BMC Pediatr. 2012 Aug 7;12:117. doi: 10.1186/1471-2431-12-117. BMC Pediatr. 2012. PMID: 22871230 Free PMC article. Clinical Trial.
References
-
- Cowett RM, Farrag HM. Selected principles of perinatal-neonatal glucose metabolism. Semin Neonatol. 2004;9(1):37–47. - PubMed
-
- Dweck HS, Cassady G. Glucose intolerance in infants of very low birth weight. I. Incidence of hyperglycemia in infants of birth weights 1,100 grams or less. Pediatrics. 1974;53(2):189–195. - PubMed
-
- Ditzenberger GR, Collins SD, Binder N. Continuous insulin intravenous infusion therapy for VLBW infants. J Perinat Neonatal Nurs. 1999;13(3):70–82. - PubMed
-
- Thabet F, Bourgeois J, Guy B, Putet G. Continuous insulin infusion in hyperglycaemic very-low-birth-weight infants receiving parenteral nutrition. Clin Nutr. 2003;22(6):545–547. - PubMed
-
- Hemachandra AH, Cowett RM. Neonatal hyperglycemia. Pediatrics Rev. 1999;20:16–24.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

