Serum insulin aspart concentrations following high-dose insulin aspart administered directly into the duodenum of healthy subjects: an open-labeled, single-blinded, and uncontrolled exploratory trial
- PMID: 20144435
- PMCID: PMC2769913
- DOI: 10.1177/193229680900300525
Serum insulin aspart concentrations following high-dose insulin aspart administered directly into the duodenum of healthy subjects: an open-labeled, single-blinded, and uncontrolled exploratory trial
Abstract
Objective: The goal of this study was to determine the bioavailability of high-dose insulin aspart administered directly into the duodenum of healthy subjects.
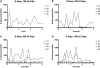
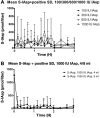
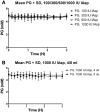
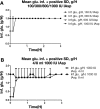
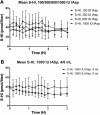
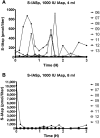
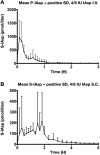
Methods: In a pilot study, four subjects each received four escalating doses of a 1-ml solution of insulin aspart (100, 300, 600, and 1000 IU, respectively) directly into the duodenum. In the following main study, eight subjects each received two identical doses of insulin aspart of 1000 IU, in 4- and 8-ml solutions, respectively, directly into the duodenum. Subjects in the main study also received an intravenous and a subcutaneous injection of 4 to 6 IU of insulin aspart.
Results: A considerable number of samples and, in some cases, consecutive samples revealed significantly increased concentrations of serum insulin aspart. Despite the significant serum insulin aspart concentrations, no significant changes of plasma glucose were measured. Moreover, no significant suppression of endogenous insulin secretion was detected, as assessed by the levels of serum human insulin.
Conclusions: Administration of high-dose insulin aspart directly into the duodenum of healthy subjects resulted in significantly increased serum insulin aspart concentrations in a high number of consecutive samples using a specific enzyme-linked immunosorbent assay. However, no significant changes in the levels of plasma glucose or serum human insulin were observed. Thus, the study did not provide any evidence of biological activity of the original insulin aspart molecule after high-dose administration directly into the duodenum.
2009 Diabetes Technology Society.
Figures
Similar articles
-
Comparative pharmacodynamic and pharmacokinetic characteristics of subcutaneous insulin glulisine and insulin aspart prior to a standard meal in obese subjects with type 2 diabetes.Diabetes Obes Metab. 2011 Mar;13(3):251-7. doi: 10.1111/j.1463-1326.2010.01343.x. Diabetes Obes Metab. 2011. PMID: 21205115 Free PMC article. Clinical Trial.
-
125I used for labelling of proteins in an absorption model changes the absorption rate of insulin aspart.Int J Pharm. 2007 Feb 7;330(1-2):114-20. doi: 10.1016/j.ijpharm.2006.09.004. Epub 2006 Sep 14. Int J Pharm. 2007. PMID: 17070660
-
Pharmacokinetics and pharmacodynamics of insulin aspart, a rapid-acting analog of human insulin, in healthy Japanese volunteers.Diabetes Res Clin Pract. 2000 Aug;49(2-3):119-26. doi: 10.1016/s0168-8227(00)00151-0. Diabetes Res Clin Pract. 2000. PMID: 10963823 Clinical Trial.
-
Clinical pharmacokinetics and pharmacodynamics of insulin aspart.Clin Pharmacokinet. 2001;40(9):641-59. doi: 10.2165/00003088-200140090-00002. Clin Pharmacokinet. 2001. PMID: 11605714 Review.
-
[Insulin aspart(NN-X14)].Nihon Rinsho. 2001 Nov;59(11):2144-50. Nihon Rinsho. 2001. PMID: 11712399 Review. Japanese.
References
-
- Heinemann L, Pfutzner A, Heise T. Alternative routes of administration as an approach to improve insulin therapy: update on dermal, oral, nasal, and pulmonary insulin delivery. Curr Pharm Design. 2001;7(14):1327–1351. - PubMed
-
- Gordan Still J. Development of oral insulin: progress and current status. Diabetes Metab Res Rev. 2002;18(Suppl.1):S29–S37. - PubMed
-
- Owens DR, Zinman B, Bolli G. Alternative routes of insulin delivery. Diabet Med. 2003;20(11):886–898. - PubMed
-
- Gwinup G, Elias AN, Vaziri ND. A case for oral insulin therapy in the prevention of micro- and macroangiopathia. Int J Artif Organs. 1990;13(7):393–395. - PubMed
-
- Porksen N, Hollingdal M, Juhl C, Butler P, Veldhuis JD, Schmitz O. Pulsatile insulin secretion: detection, regulation, and role in diabetes. Diabetes. 2002;51(Suppl 1):S245–S254. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

