The effect of stereochemistry on the thermodynamic characteristics of the binding of fenoterol stereoisomers to the beta(2)-adrenoceptor
- PMID: 20144591
- PMCID: PMC2850566
- DOI: 10.1016/j.bcp.2010.01.035
The effect of stereochemistry on the thermodynamic characteristics of the binding of fenoterol stereoisomers to the beta(2)-adrenoceptor
Abstract
The binding thermodynamics of the stereoisomers of fenoterol, (R,R')-, (S,S')-, (R,S')-, and (S,R')-fenoterol, to the beta(2)-adrenergic receptor (beta(2)-AR) have been determined. The experiments utilized membranes obtained from HEK cells stably transfected with cDNA encoding human beta(2)-AR. Competitive displacement studies using [(3)H]CGP-12177 as the marker ligand were conducted at 4, 15, 25, 30 and 37 degrees C, the binding affinities calculated and the standard enthalpic (DeltaH degrees ) and standard entropic (DeltaS degrees ) contribution to the standard free energy change (DeltaG degrees ) associated with the binding process determined through the construction of van't Hoff plots. The results indicate that the binding of (S,S')- and (S,R')-fenoterol were predominately enthalpy-driven processes while the binding of (R,R')- and (R,S')-fenoterol were entropy-driven. All of the fenoterol stereoisomers are full agonists of the beta(2)-AR, and, therefore, the results of this study are inconsistent with the previously described "thermodynamic agonist-antagonist discrimination", in which the binding of an agonist to the beta-AR is entropy-driven and the binding of an antagonist is enthalpy-driven. In addition, the data demonstrate that the chirality of the carbon atom containing the beta-hydroxyl group of the fenoterol molecule (the beta-OH carbon) is a key factor in the determination of whether the binding process will be enthalpy-driven or entropy-driven. When the configuration at the beta-OH carbon is S the binding process is enthalpy-driven while the R configuration produces an entropy-driven process.
Published by Elsevier Inc.
Figures

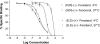
 ), (S,R')-fenoterol (
), (S,R')-fenoterol ( ), (R,S')-fenoterol (
), (R,S')-fenoterol ( ), (R,R')-fenoterol (
), (R,R')-fenoterol ( ), rac-propranolol (∎) , (R)-isoproterenol (▴). (Black and White print version) Van't Hoff plots for (S,S')-fenoterol (●), (S,R')-fenoterol (○), (R,S')-fenoterol (◆), (R,R')-fenoterol (◊), rac-propranolol (∎) , (R)-isoproterenol (▴).
), rac-propranolol (∎) , (R)-isoproterenol (▴). (Black and White print version) Van't Hoff plots for (S,S')-fenoterol (●), (S,R')-fenoterol (○), (R,S')-fenoterol (◆), (R,R')-fenoterol (◊), rac-propranolol (∎) , (R)-isoproterenol (▴).
 ), (S,R')-fenoterol (
), (S,R')-fenoterol ( ), (R,S')-fenoterol (
), (R,S')-fenoterol ( ), (R,R')-fenoterol (
), (R,R')-fenoterol ( ), rac-propranolol (∎) , (R)-isoproterenol (▴). (Black and White print version) Van't Hoff plots for (S,S')-fenoterol (●), (S,R')-fenoterol (○), (R,S')-fenoterol (◆), (R,R')-fenoterol (◊), rac-propranolol (∎) , (R)-isoproterenol (▴).
), rac-propranolol (∎) , (R)-isoproterenol (▴). (Black and White print version) Van't Hoff plots for (S,S')-fenoterol (●), (S,R')-fenoterol (○), (R,S')-fenoterol (◆), (R,R')-fenoterol (◊), rac-propranolol (∎) , (R)-isoproterenol (▴).
 ), (S,R')-fenoterol (
), (S,R')-fenoterol ( ), (R,S')-fenoterol (
), (R,S')-fenoterol ( ), (R,R')-fenoterol (
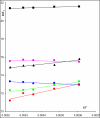
), (R,R')-fenoterol ( ), rac-propranolol (∎), (R)-isoproterenol (▴). The plot indicates also the location determined for rac-fenoterol (
), rac-propranolol (∎), (R)-isoproterenol (▴). The plot indicates also the location determined for rac-fenoterol ( ) by Weiland et.al [1]. (Black and White print version) Enthalpy/entropy relation plot for (S,S')-fenoterol (●), (S,R')-fenoterol (○), (R,S')-fenoterol (◆), (R,R')-fenoterol (◊), rac-propranolol (∎) , (R)-isoproterenol (▴). The plot indicates also the location determined for rac-fenoterol (
) by Weiland et.al [1]. (Black and White print version) Enthalpy/entropy relation plot for (S,S')-fenoterol (●), (S,R')-fenoterol (○), (R,S')-fenoterol (◆), (R,R')-fenoterol (◊), rac-propranolol (∎) , (R)-isoproterenol (▴). The plot indicates also the location determined for rac-fenoterol ( ) by Weiland et.al [1].
) by Weiland et.al [1].
 ), (S,R')-fenoterol (
), (S,R')-fenoterol ( ), (R,S')-fenoterol (
), (R,S')-fenoterol ( ), (R,R')-fenoterol (
), (R,R')-fenoterol ( ), rac-propranolol (∎), (R)-isoproterenol (▴). The plot indicates also the location determined for rac-fenoterol (
), rac-propranolol (∎), (R)-isoproterenol (▴). The plot indicates also the location determined for rac-fenoterol ( ) by Weiland et.al [1]. (Black and White print version) Enthalpy/entropy relation plot for (S,S')-fenoterol (●), (S,R')-fenoterol (○), (R,S')-fenoterol (◆), (R,R')-fenoterol (◊), rac-propranolol (∎) , (R)-isoproterenol (▴). The plot indicates also the location determined for rac-fenoterol (
) by Weiland et.al [1]. (Black and White print version) Enthalpy/entropy relation plot for (S,S')-fenoterol (●), (S,R')-fenoterol (○), (R,S')-fenoterol (◆), (R,R')-fenoterol (◊), rac-propranolol (∎) , (R)-isoproterenol (▴). The plot indicates also the location determined for rac-fenoterol ( ) by Weiland et.al [1].
) by Weiland et.al [1].Similar articles
-
Thermodynamics and docking of agonists to the β(2)-adrenoceptor determined using [(3)H](R,R')-4-methoxyfenoterol as the marker ligand.Mol Pharmacol. 2012 Jun;81(6):846-54. doi: 10.1124/mol.111.077347. Epub 2012 Mar 20. Mol Pharmacol. 2012. PMID: 22434858 Free PMC article.
-
Effect of fenoterol stereochemistry on the β2 adrenergic receptor system: ligand-directed chiral recognition.Chirality. 2011;23 Suppl 1(Suppl 1):E1-6. doi: 10.1002/chir.20963. Epub 2011 May 26. Chirality. 2011. PMID: 21618615 Free PMC article. Review.
-
Comparative molecular field analysis of fenoterol derivatives interacting with an agonist-stabilized form of the β₂-adrenergic receptor.Bioorg Med Chem. 2014 Jan 1;22(1):234-46. doi: 10.1016/j.bmc.2013.11.030. Epub 2013 Nov 23. Bioorg Med Chem. 2014. PMID: 24326276 Free PMC article.
-
Enantioselective separation and online affinity chromatographic characterization of R,R- and S,S-fenoterol.Chirality. 2006 Nov;18(10):822-7. doi: 10.1002/chir.20317. Chirality. 2006. PMID: 16917835
-
Can thermodynamic measurements of receptor binding yield information on drug affinity and efficacy?Biochem Pharmacol. 2000 Dec 1;60(11):1549-56. doi: 10.1016/s0006-2952(00)00368-3. Biochem Pharmacol. 2000. PMID: 11077036 Review.
Cited by
-
{Beta}2-adrenergic receptor agonists inhibit the proliferation of 1321N1 astrocytoma cells.J Pharmacol Exp Ther. 2011 Feb;336(2):524-32. doi: 10.1124/jpet.110.173971. Epub 2010 Nov 11. J Pharmacol Exp Ther. 2011. PMID: 21071556 Free PMC article.
-
Thermodynamics and docking of agonists to the β(2)-adrenoceptor determined using [(3)H](R,R')-4-methoxyfenoterol as the marker ligand.Mol Pharmacol. 2012 Jun;81(6):846-54. doi: 10.1124/mol.111.077347. Epub 2012 Mar 20. Mol Pharmacol. 2012. PMID: 22434858 Free PMC article.
-
Interaction of fenoterol stereoisomers with β2-adrenoceptor-G sα fusion proteins: antagonist and agonist competition binding.Naunyn Schmiedebergs Arch Pharmacol. 2015 May;388(5):517-24. doi: 10.1007/s00210-015-1086-5. Epub 2015 Jan 31. Naunyn Schmiedebergs Arch Pharmacol. 2015. PMID: 25637582
-
The stereoselective sulfate conjugation of 4'-methoxyfenoterol stereoisomers by sulfotransferase enzymes.Chirality. 2012 Oct;24(10):796-803. doi: 10.1002/chir.22072. Epub 2012 Jun 29. Chirality. 2012. PMID: 22744891 Free PMC article.
-
Molecular interactions between fenoterol stereoisomers and derivatives and the β₂-adrenergic receptor binding site studied by docking and molecular dynamics simulations.J Mol Model. 2013 Nov;19(11):4919-30. doi: 10.1007/s00894-013-1981-y. Epub 2013 Sep 17. J Mol Model. 2013. PMID: 24043542 Free PMC article.
References
-
- Weiland GA, Minneman KP, Molinoff PB. Fundamental difference between the molecular interactions of agonists and antagonists with the β-adrenergic receptor. Nature. 1979;281:114–117. - PubMed
-
- Contreras ML, Wolfe BB, Molinoff PB. Thermodynamic properties of agonist interactions with the beta adrenergic receptor-coupled adenylate cyclase system. I. high- and low-affinity states of agonist binding to membrane-bound beta adrenergic receptors. J Pharm Exp Ther. 1986;237:154–64. - PubMed
-
- Miklavc A, Kocjan D, Mavri J, Koller J, Hadzi D. On the fundamental difference in the thermodynamics of agonist and antagonist interactions with β-adrenergic receptors and the mechanism of entropy-driven binding. Biochem Pharm. 1990;40:663–9. - PubMed
-
- Borea PA, Dalpiaz A, Varani K, Gilli P, Gilli G. Can Thermodynamic Measurements of Receptor Binding Yield Information on Drug Affinity and Efficacy? Biochem Pharm. 2000;60:1549–1556. - PubMed
-
- Merighi S, Simioni C, Gessi S, Varani K, Borea PA. Binding thermodynamics at the human cannabinoid CB1 and CB2 receptors. Biochem Pharmacol. 2010;79:471–77. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

