Progesterone inhibits apoptosis in part by PGRMC1-regulated gene expression
- PMID: 20144686
- PMCID: PMC2844455
- DOI: 10.1016/j.mce.2010.02.005
Progesterone inhibits apoptosis in part by PGRMC1-regulated gene expression
Abstract
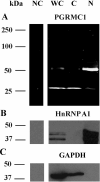
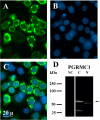
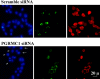
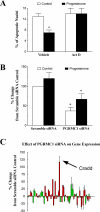
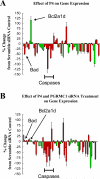
Progesterone receptor membrane component-1 (PGRMC1) is present in both the cytoplasm and nucleus of spontaneously immortalized granulosa cells (SIGCs). PGRMC1 is detected as a monomer in the cytoplasm and a DTT-resistant PGRMC1 dimer in the nucleus. Transfected PGRMC1-GFP localizes mainly to the cytoplasm and does not form a DTT-resistant dimer. Moreover, forced expression of PGRMC1-GFP increases the sensitivity of the SIGCs to progesterone (P4)'s anti-apoptotic action, indicating that the PGRMC1 monomer is functional. However, when endogenous PGRMC1 is depleted by siRNA treatment and replaced with PGRMC1-GFP, P4 responsiveness is not enhanced, although overall levels of PGRMC1 are increased. P4's anti-apoptotic action is also attenuated by actinomycin D, an inhibitor of RNA synthesis, and P4 activation of PGRMC1 suppresses Bad and increases Bcl2a1d expression. Taken together, the present studies suggest a genomic component to PGRMC1's anti-apoptotic mechanism of action, which requires the presence of the PGRMC1 dimer.
(c) 2010 Elsevier Ireland Ltd. All rights reserved.
Figures



Similar articles
-
Progesterone regulation of progesterone receptor membrane component 1 (PGRMC1) sumoylation and transcriptional activity in spontaneously immortalized granulosa cells.Endocrinology. 2012 Aug;153(8):3929-39. doi: 10.1210/en.2011-2096. Epub 2012 Jun 19. Endocrinology. 2012. PMID: 22719051 Free PMC article.
-
Progesterone receptor membrane component-1 (PGRMC1) is the mediator of progesterone's antiapoptotic action in spontaneously immortalized granulosa cells as revealed by PGRMC1 small interfering ribonucleic acid treatment and functional analysis of PGRMC1 mutations.Endocrinology. 2008 Feb;149(2):534-43. doi: 10.1210/en.2007-1050. Epub 2007 Nov 8. Endocrinology. 2008. PMID: 17991724 Free PMC article.
-
Plasminogen activator inhibitor 1 RNA-binding protein interacts with progesterone receptor membrane component 1 to regulate progesterone's ability to maintain the viability of spontaneously immortalized granulosa cells and rat granulosa cells.Biol Reprod. 2013 Jan 25;88(1):20. doi: 10.1095/biolreprod.112.103036. Print 2013 Jan. Biol Reprod. 2013. PMID: 23242527 Free PMC article.
-
Evidence for a genomic mechanism of action for progesterone receptor membrane component-1.Steroids. 2012 Aug;77(10):1007-12. doi: 10.1016/j.steroids.2012.01.013. Epub 2012 Feb 1. Steroids. 2012. PMID: 22326699 Free PMC article. Review.
-
Non-canonical progesterone signaling in granulosa cell function.Reproduction. 2014 Apr 8;147(5):R169-78. doi: 10.1530/REP-13-0582. Print 2014 May. Reproduction. 2014. PMID: 24516175 Free PMC article. Review.
Cited by
-
Progesterone regulation of progesterone receptor membrane component 1 (PGRMC1) sumoylation and transcriptional activity in spontaneously immortalized granulosa cells.Endocrinology. 2012 Aug;153(8):3929-39. doi: 10.1210/en.2011-2096. Epub 2012 Jun 19. Endocrinology. 2012. PMID: 22719051 Free PMC article.
-
Expression of progesterone receptor membrane component-1 in bovine reproductive system during estrous cycle.Eur J Histochem. 2011;55(3):e27. doi: 10.4081/ejh.2011.e27. Epub 2011 Aug 27. Eur J Histochem. 2011. PMID: 22073374 Free PMC article.
-
PGRMC1 Elevation in Multiple Cancers and Essential Role in Stem Cell Survival.Adv Lung Cancer (Irvine). 2015 Sep;4(3):37-51. doi: 10.4236/alc.2015.43006. Epub 2016 Jan 28. Adv Lung Cancer (Irvine). 2015. PMID: 27867772 Free PMC article.
-
Insights on the Role of PGRMC1 in Mitotic and Meiotic Cell Division.Cancers (Basel). 2022 Nov 23;14(23):5755. doi: 10.3390/cancers14235755. Cancers (Basel). 2022. PMID: 36497237 Free PMC article. Review.
-
Progesterone Receptor Membrane Component 1 suppresses the p53 and Wnt/β-catenin pathways to promote human pluripotent stem cell self-renewal.Sci Rep. 2018 Feb 14;8(1):3048. doi: 10.1038/s41598-018-21322-z. Sci Rep. 2018. PMID: 29445107 Free PMC article.
References
-
- Ahmad M, Srinivasula SM, Wang L, Talanian RV, Litwack G, Fernandes-Alnemri T, Alnemri ES. CRADD, a novel human apoptotic adaptor molecule for caspase-2, and FasL/tumor necrosis factor receptor-interacting protein RIP. Cancer Res. 1997;57:615–619. - PubMed
-
- Cahill MA. Progesterone receptor membrane component 1: an integrative review. J Steroid Biochem Mol Biol. 2007;105:16–36. - PubMed
-
- Conneely OM, Lydon JP. Progesterone receptors in reproduction: functional impact of the A and B isoforms. Steroids. 2000;65:571–577. - PubMed
-
- Crudden G, Chitti RE, Craven RJ. Hpr6 (heme-1 domain protein) regulates the susceptibility of cancer cells to chemotherapeutic drugs. J Pharmacol Exp Ther. 2006;316:448–455. - PubMed
-
- Engmann L, Losel R, Wehling M, Peluso JJ. Progesterone regulation of human granulosa/luteal cell viability by an RU486-independent mechanism. J Clin Endocrinol Metab. 2006;91:4962–4968. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

