Regulation of axonal trafficking of cytochrome c oxidase IV mRNA
- PMID: 20144716
- PMCID: PMC2845174
- DOI: 10.1016/j.mcn.2010.01.009
Regulation of axonal trafficking of cytochrome c oxidase IV mRNA
Abstract
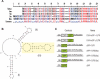
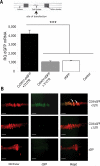
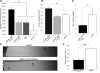
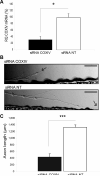
Trafficking and local translation of axonal mRNAs play a critical role in the development and function of this neuronal subcellular structural domain. In this report, we studied cytochrome c oxidase subunit IV (COXIV) mRNA trafficking into distal axons of primary superior cervical ganglia (SCG) neurons, and provided evidence that axonal trafficking and mitochondrial association of the mRNA are mediated by an element located in a 38bp-long, hairpin-loop forming region within the 3'UTR of the transcript. Our results also suggest that suppression of local translation of COXIV mRNA results in significant attenuation of axonal elongation. Taken together, the results provide the first evidence for the existence of a cis-acting axonal transport element within a nuclear-encoding mitochondrial gene, and demonstrate the importance of the axonal trafficking and local translation of nuclear-encoded mitochondrial mRNAs in axonal growth.
Published by Elsevier Inc.
Figures

References
-
- Bassell G, Singer RH. mRNA and cytoskeletal filaments. Curr. Opin. Cell Biol. 1997;9:109–115. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

