Perivascular nitric oxide activates notch signaling and promotes stem-like character in PDGF-induced glioma cells
- PMID: 20144787
- PMCID: PMC3818090
- DOI: 10.1016/j.stem.2010.01.001
Perivascular nitric oxide activates notch signaling and promotes stem-like character in PDGF-induced glioma cells
Abstract
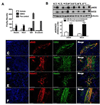
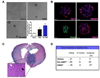
eNOS expression is elevated in human glioblastomas and correlated with increased tumor growth and aggressive character. We investigated the potential role of nitric oxide (NO) activity in the perivascular niche (PVN) using a genetic engineered mouse model of PDGF-induced gliomas. eNOS expression is highly elevated in tumor vascular endothelium adjacent to perivascular glioma cells expressing Nestin, Notch, and the NO receptor, sGC. In addition, the NO/cGMP/PKG pathway drives Notch signaling in PDGF-induced gliomas in vitro, and induces the side population phenotype in primary glioma cell cultures. NO also increases neurosphere forming capacity of PDGF-driven glioma primary cultures, and enhances their tumorigenic capacity in vivo. Loss of NO activity in these tumors suppresses Notch signaling in vivo and prolongs survival of mice. This mechanism is conserved in human PDGFR amplified gliomas. The NO/cGMP/PKG pathway's promotion of stem cell-like character in the tumor PVN may identify therapeutic targets for this subset of gliomas.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures




Comment in
-
NO Signals from the Cancer Stem Cell Niche.Cell Stem Cell. 2010 Feb 5;6(2):97-8. doi: 10.1016/j.stem.2010.01.007. Cell Stem Cell. 2010. PMID: 20144782
References
-
- Bakshi A, Nag TC, Wadhwa S, Mahapatra AK, Sarkar C. The expression of nitric oxide synthases in human brain tumours and peritumoral areas. J Neurol Sci. 1998;155(2):196–203. - PubMed
-
- Becher OJ, Hambardzumyan D, Fomchenko EI, Momota H, Mainwaring L, Bleau AM, Katz AM, Edgar M, Kenney AM, Cordon-Cardo C, et al. Gli activity correlates with tumor grade in platelet-derived growth factor-induced gliomas. Cancer Res. 2008;68(7):2241–2249. - PubMed
-
- Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev. 2006;58(3):488–520. - PubMed
-
- Blaise GA, Gauvin D, Gangal M, Authier S. Nitric oxide, cell signaling and cell death. Toxicology. 2005;208(2):177–192. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

