Strong dependence between functional domains in a dual-function snoRNA infers coupling of rRNA processing and modification events
- PMID: 20144950
- PMCID: PMC2879522
- DOI: 10.1093/nar/gkq043
Strong dependence between functional domains in a dual-function snoRNA infers coupling of rRNA processing and modification events
Abstract
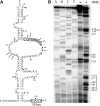
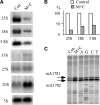
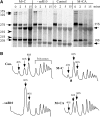
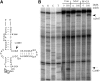
Most small nucleolar RNAs (snoRNAs) guide rRNA nucleotide modifications, some participate in pre-rRNA cleavages, and a few have both functions. These activities involve direct base-pairing of the snoRNA with pre-rRNA using different domains. It is not known if the modification and processing functions occur independently or in a coordinated manner. We address this question by mutational analysis of a yeast box H/ACA snoRNA that mediates both processing and modification. This snoRNA (snR10) contains canonical 5'- and 3'-hairpin structures with a guide domain for pseudouridylation in the 3' hairpin. Our functional mapping results show that: (i) processing requires the 5' hairpin exclusively, in particular a 7-nt element; (ii) loss of the 3' hairpin or pseudouridine does not affect rRNA processing; (iii) a single nucleotide insertion in the guide domain shifts modification to an adjacent uridine in rRNA, and severely impairs both processing and cell growth; and (iv) the deleterious effects of the insertion mutation depend on the presence of the processing element in the 5' hairpin, but not modification of the novel site. Together, the results suggest that the snoRNA hairpins function in a coordinated manner and that their interactions with pre-rRNA could be coupled.
Figures





Similar articles
-
Genome-wide analysis of small nucleolar RNAs of Leishmania major reveals a rich repertoire of RNAs involved in modification and processing of rRNA.RNA Biol. 2015;12(11):1222-55. doi: 10.1080/15476286.2015.1038019. Epub 2015 May 13. RNA Biol. 2015. PMID: 25970223 Free PMC article.
-
Trypanosoma brucei 5'ETS A'-cleavage is directed by 3'-adjacent sequences, but not two U3 snoRNA-binding elements, which are all required for subsequent pre-small subunit rRNA processing events.J Mol Biol. 2001 Nov 2;313(4):733-49. doi: 10.1006/jmbi.2001.5078. J Mol Biol. 2001. PMID: 11697900
-
Small RNAs with big implications: new insights into H/ACA snoRNA function and their role in human disease.Wiley Interdiscip Rev RNA. 2015 Mar-Apr;6(2):173-89. doi: 10.1002/wrna.1266. Epub 2014 Oct 31. Wiley Interdiscip Rev RNA. 2015. PMID: 25363811 Free PMC article. Review.
-
Comparative structure analysis of vertebrate U17 small nucleolar RNA (snoRNA).J Mol Evol. 2002 Feb;54(2):166-79. doi: 10.1007/s00239-001-0065-2. J Mol Evol. 2002. PMID: 11821910
-
SnoRNAs as tools for RNA cleavage and modification.Nucleic Acids Symp Ser. 1997;(36):61-3. Nucleic Acids Symp Ser. 1997. PMID: 9478207 Review.
Cited by
-
A pre-ribosomal RNA interaction network involving snoRNAs and the Rok1 helicase.RNA. 2014 Aug;20(8):1173-82. doi: 10.1261/rna.044669.114. Epub 2014 Jun 19. RNA. 2014. PMID: 24947498 Free PMC article.
-
The multistructural forms of box C/D ribonucleoprotein particles.RNA. 2018 Dec;24(12):1625-1633. doi: 10.1261/rna.068312.118. Epub 2018 Sep 25. RNA. 2018. PMID: 30254138 Free PMC article. Review.
-
Stepwise and dynamic assembly of the earliest precursors of small ribosomal subunits in yeast.Genes Dev. 2016 Mar 15;30(6):718-32. doi: 10.1101/gad.274688.115. Genes Dev. 2016. PMID: 26980190 Free PMC article.
-
Passing the anaerobic threshold is associated with substantial changes in the gene expression profile in white blood cells.Eur J Appl Physiol. 2012 Mar;112(3):963-72. doi: 10.1007/s00421-011-2048-3. Epub 2011 Jun 30. Eur J Appl Physiol. 2012. PMID: 21717121 Clinical Trial.
-
Yeast Rrp8p, a novel methyltransferase responsible for m1A 645 base modification of 25S rRNA.Nucleic Acids Res. 2013 Jan;41(2):1151-63. doi: 10.1093/nar/gks1102. Epub 2012 Nov 23. Nucleic Acids Res. 2013. PMID: 23180764 Free PMC article.
References
-
- Nazar RN. Ribosomal RNA processing and ribosome biogenesis in eukaryotes. IUBMB Life. 2004;56:457–465. - PubMed
-
- Warner JR. Nascent ribosomes. Cell. 2001;107:133–136. - PubMed
-
- Fatica A, Tollervey D. Making ribosomes. Curr. Opin. Cell Biol. 2002;14:313–318. - PubMed
-
- Bachellerie JP, Cavaille J, Huttenhofer A. The expanding snoRNA world. Biochimie. 2002;84:775–790. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

