Control of mitotic and meiotic centriole duplication by the Plk4-related kinase ZYG-1
- PMID: 20144993
- PMCID: PMC2823580
- DOI: 10.1242/jcs.050682
Control of mitotic and meiotic centriole duplication by the Plk4-related kinase ZYG-1
Abstract
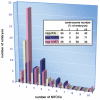
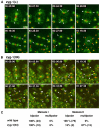
Centriole duplication is of crucial importance during both mitotic and male meiotic divisions, but it is currently not known whether this process is regulated differently during the two modes of division. In Caenorhabditis elegans, the kinase ZYG-1 plays an essential role in both mitotic and meiotic centriole duplication. We have found that the C-terminus of ZYG-1 is necessary and sufficient for targeting to centrosomes and is important for differentiating mitotic and meiotic centriole duplication. Small truncations of the C-terminus dramatically lower the level of ZYG-1 at mitotic centrosomes but have little effect on the level of ZYG-1 at meiotic centrosomes. Interestingly, truncation of ZYG-1 blocks centrosome duplication in the mitotic cycle but leads to centrosome amplification in the meiotic cycle. Meiotic centriole amplification appears to result from the overduplication of centrioles during meiosis I and leads to the formation of multipolar meiosis II spindles. The extra centrioles also disrupt spermatogenesis by inducing the formation of supernumerary fertilization-competent spermatids that contain abnormal numbers of chromosomes and centrioles. Our data reveal differences in the regulation of mitotic and meiotic centrosome duplication, particularly with regard to ZYG-1 activity, and reveal an important role for centrosomes in spermatid formation.
Figures



References
-
- Albertson D. G., Thomson J. N. (1993). Segregation of holocentric chromosomes at meiosis in the nematode, Caenorhabditis elegans. Chromosome Res. 1, 15-26 - PubMed
-
- Basto R., Lau J., Vinogradova T., Gardiol A., Woods C. G., Khodjakov A., Raff J. W. (2006). Flies without centrioles. Cell 125, 1375-1386 - PubMed
-
- Bettencourt-Dias M., Rodrigues-Martins A., Carpenter L., Riparbelli M., Lehmann L., Gatt M. K., Carmo N., Balloux F., Callaini G., Glover D. M. (2005). SAK/PLK4 is required for centriole duplication and flagella development. Curr. Biol. 15, 2199-2207 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

