Molecular mechanisms of protein and lipid targeting to ciliary membranes
- PMID: 20145001
- PMCID: PMC2818192
- DOI: 10.1242/jcs.062968
Molecular mechanisms of protein and lipid targeting to ciliary membranes
Abstract
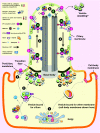
Cilia are specialized surface regions of eukaryotic cells that serve a variety of functions, ranging from motility to sensation and to regulation of cell growth and differentiation. The discovery that a number of human diseases, collectively known as ciliopathies, result from defective cilium function has expanded interest in these structures. Among the many properties of cilia, motility and intraflagellar transport have been most extensively studied. The latter is the process by which multiprotein complexes associate with microtubule motors to transport structural subunits along the axoneme to and from the ciliary tip. By contrast, the mechanisms by which membrane proteins and lipids are specifically targeted to the cilium are still largely unknown. In this Commentary, we review the current knowledge of protein and lipid targeting to ciliary membranes and outline important issues for future study. We also integrate this information into a proposed model of how the cell specifically targets proteins and lipids to the specialized membrane of this unique organelle.
Figures
References
-
- Adhiambo C., Blisnick T., Toutirais G., Delannoy E., Bastin P. (2009). A novel function for the atypical small G protein Rab-like 5 in the assembly of the trypanosome flagellum. J. Cell Sci. 122, 834-841 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

